News & Media-2020
2020
Home / News & Media / 2020
2020
NEWS & MEDIA:

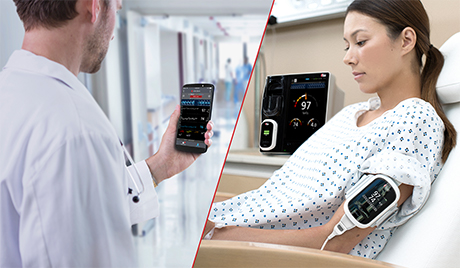
Masimo Helps Clinicians at Renown Health Manage the COVID-19 Surge
Irvine, California – December 21, 2020 – Masimo (NASDAQ: MASI) announced today that Masimo is helping clinicians at Renown Health in Reno, Nevada, to address the COVID-19 surge, including through use of Masimo SafetyNet™, a remote patient management platform. Renown Health is using Masimo SafetyNet to help care for patients positive for COVID-19 recover at home – and to help notify clinicians of the need to intervene when patients exhibit signs of deterioration.
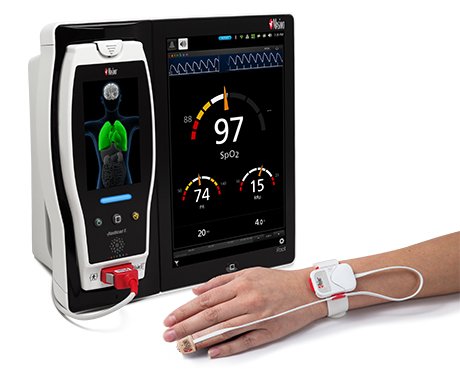
Renown Health is a non-profit integrated healthcare network serving Nevada, Lake Tahoe, and northeast California. Masimo SafetyNet, introduced earlier this year as part of Masimo’s efforts to help hospitals and clinicians combat the pandemic, is now in use at numerous institutions around the world. Renown Health uses Masimo Patient SafetyNet™ and Masimo Rad-97® Pulse CO-Oximeters® to provide bedside and remote oxygen saturation and respiratory and pulse rate monitoring and notification within their hospitals and at a state-of-the-art 700-bed Alternate Care Facility. The facility has expanded into an adjacent parking structure, turned into a makeshift general ward to accommodate the unprecedented surge of patients suffering from COVID-19. In addition, Renown was one of the first systems to implement Masimo SafetyNet to expand patient care from the hospital to the home.
"We at Renown are proud of our national reputation as an innovator in implementing new models, technology, and systems of care for the community,” said Tony Slonim, MD, DrPH, President & CEO. "We are working with some incredible partners, including Masimo, to transform care and demonstrate value in a way that appeals to patients and helps clinicians improve outcomes and reduce costs. Renown's Hospital-at-Home model of care technology enables patients to receive hospital-level care in the comfort of their own homes. This is especially critical for patients who are elderly, frail or vulnerable to complications."
"This type of technology is a game-changer in improving patient care," said Paul Sierzenski, MD, MSHQS, Chief Medical Officer, Acute Services, Renown Health. "Given the demand on our hospitals during this COVID-19 pandemic, we are pleased to provide appropriate patients with this telehealth solution, which assists in our management of patients with COVID-19 and allows patients the convenience of being in their own homes."
Renown has found particular benefit in using Masimo SafetyNet to spot possible deterioration in home-based patients' vital signs and intervene proactively, before their condition worsens. Dr. David Lemak, Section Chief for Urgent Care at Renown Medical Group-Ryland St., heads the team which conducts virtual telehealth visits with discharged patients. Recently, they noticed several drops in the oxygen saturation of Jim Cox, a 58-year-old patient who was being monitored at home as he recovered from COVID-19. Following a virtual visit with Dr. Lemak, Jim returned to the hospital, where cardiac complications could be addressed.
"I was excited to be one of the first patients chosen to use the Masimo SafetyNet 'hospital-at-home' technology," said Jim Cox of Reno. "The system was easy to use. I was able to monitor my own vital signs through the app on my cell phone, and was pleased with the virtual medical visits provided by Dr. Lemak, and the nurses monitoring my device. It was a relief to be back with my family, recuperating in my own bed and making my own meals."
"With Masimo SafetyNet," said Dr. Lemak, "we have access to continuous, meaningful data, without burdening our recovering patients, giving my team confidence that even when they're not physically present with us, our patients are in good hands. We're so grateful to have been able to help Jim – without this system, the outcome could have been very different." Jim added, "While I was at home, my medical condition did not improve. Without this device and the Renown staff checking in with me, I probably would have ignored my symptoms and tried to sleep it off. Instead, I was immediately admitted back to the hospital."
"Our patients at Renown have the most sophisticated and reliable respiratory monitoring available anywhere," said Jason Farnsworth, MBA, Director of Respiratory Care Services at Renown. "We know that physiologic monitoring improves outcomes and saves lives. The ability to extend that capability to patients in non-traditional settings and at home during this crisis is transformative. Our teams are excited to be able to help patients and their families – and ensure they receive the clinical support they need by using this technology and monitoring."
"To know that we can provide quality care for patients recovering from COVID-19, and help ease their transition back to their normal lives, where they can recover in the comfort of their own homes, is fantastic," said Mitchell Fong, Director of Telehealth Services at Renown. "Patients have shared that they enjoy being at home, sitting in their favorite recliner, enjoying a home-cooked meal and even transitioning back to remote work. If we can help people feel better, we feel better."
Joe Kiani, Founder and CEO of Masimo, said, "We are grateful to be able to partner with institutions like Renown Health to make a positive difference at this exceptionally difficult time. Like hospitals and doctors everywhere, Renown is doing their best to help as many patients as possible, and Masimo SafetyNet allows them to stretch their resources and keep much-in-demand in-hospital beds free for the most acute cases, without compromising their ability to keep track of lower-acuity patients efficiently and effectively at home. We look forward to continuing to collaborate with Renown Health in the future as we expand the ways that Masimo SafetyNet and our other monitoring and automation solutions can help Renown improve their patient care and outcomes."
Harnessing its expertise in noninvasive patient monitoring, automation, and telehealth solutions, Masimo developed Masimo SafetyNet – which combines hospital-grade tetherless pulse oximetry, continuous temperature measurement, and a remote data capture and surveillance platform – to help hospitals accommodate surges in COVID-19 patient volume and help lower-acuity patients recover at home and in other alternate care spaces. Masimo SafetyNet allows clinicians to monitor their patients' physiological status – including oxygen saturation, respiration rate, pulse rate, and temperature – from afar, at all times. Masimo SafetyNet can be rapidly deployed and easily scaled as needed, without additional hardware or network infrastructure, helping caregivers provide the best possible care during this pandemic.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
About Renown Health
Renown Health is a locally governed and locally owned, not-for-profit integrated healthcare network serving northern Nevada, Lake Tahoe and northeast California. Renown is one of the region's largest private employers with a workforce of more than 7,000. It comprises three acute care hospitals, a rehabilitation hospital, the area's most comprehensive medical group and urgent care network, and the region's largest and only locally owned not-for-profit insurance company, Hometown Health. Renown has a long tradition and commitment to continually improve the care and the health of our community. For more information, visit renown.org. most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Renown Public Relations
Phone: (775) 691-7308
Email: [email protected]

Researchers Use Masimo ORi™, Oxygen Reserve Index, to Help Suppress Postoperative Hyperoxia in Patients Undergoing Breast Surgery
Neuchatel, Switzerland – December 14, 2020 – Masimo (NASDAQ: MASI) announced today the findings of an abstract recently presented at Euroanaesthesia 2020 in which Dr. Kumagai and colleagues at Iwate Medical University Hospital in Yahaba-cho, Japan investigated whether Masimo ORi™, Oxygen Reserve Index, could be used to limit the extent of postoperative hyperoxia.1 The researchers concluded that ORi helped “suppress hyperoxia, preventing hypoxia.”
ORi, available outside the U.S., is a noninvasive and continuous parameter intended to provide insight into a patient’s oxygen status during moderate hyperoxia. Enabled by the multi-wavelength rainbow® Pulse CO-Oximetry platform, ORi is provided alongside oxygen saturation (SpO2), a clinically proven Masimo SET® pulse oximetry measurement.
Noting that postoperative hyperoxia is associated with various adverse outcomes—including “acute lung injury, increased hospital mortality, and worse outcomes in patients with ischemic stroke”—the researchers sought to determine whether a noninvasive, continuous parameter could help clinicians assess the appropriate amount of supplemental oxygen to administer to surgical patients, so as to limit hyperoxia post-surgery. They divided 50 patients scheduled for breast surgery into a group receiving ORi-based oxygen treatment (group O) and a control group that received conventional postoperative oxygen treatment (group C). In group C, after extubation, oxygen was administered at a fixed rate (4 L/min); in group O, oxygen was administered at 4 L/min but decreased by 0.5 L/min if ORi > 0.00, until ORi was 0.00 for 30 minutes continuously. Blood gas analysis was performed at various intervals. Hyperoxia was defined as partial pressure of arterial oxygen (PaO2) > 120 mmHg, and hypoxia as oxygen saturation (SpO2) ≤ 94% for more than 1 minute.
The researchers found that PaO2 was significantly lower in the ORi group both before patients left the PACU (mean 117.3 mmHg [one standard deviation 26.8 mmHg] in group O vs. 170.0 mmHg [42.8 mmHg] in group C) and the morning after surgery (107.5 mmHg [16.5 mmHg] in group O vs. 157.1 mmHg [28.4 mmHg] in group C); p < 0.01. No patients had hypoxia.
The researchers concluded, “Determining postoperative supplemental oxygen amount using ORi can noninvasively suppress hyperoxia, preventing hypoxia.”
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2020-21 >U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Kumagai M, Koishi W, Kurihara H, Eizuka A, Suzuki K. Contribution of the novel pulse oximeter-based index in determining the amount of postoperative supplemental oxygen needed. Proceedings from the Euroanaesthesia 2020 Annual Meeting. #4339.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at http://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302..
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Study Associates Masimo SpHb®, Noninvasive and Continuous Hemoglobin Monitoring, as Part of Pediatric Patient Blood Management, with Reduced ICU Stays and Postoperative Transfusion
Neuchatel, Switzerland – December 10, 2020 – Masimo (NASDAQ: MASI) announced today the findings of an abstract recently presented at Euroanaesthesia 2020 in which Dr. Saraçoğlu and colleagues at Marmara University in Istanbul, Turkey investigated the efficacy of Masimo noninvasive and continuous hemoglobin monitoring, SpHb®, as part of the transfusion management of pediatric patients undergoing major surgery.1 The researchers found that use of SpHb was associated with decreased rate of postoperative transfusion, reduced length of ICU stay, and other improved outcomes.
Noting that traditional methods of measuring hemoglobin and estimating blood loss as part of perioperative blood transfusion management are "time consuming" and may cause delays in decision making, the researchers sought to investigate whether use of a noninvasive, continuous method, Masimo SpHb, would have an impact on transfusion rates, morbidity, and mortality in pediatric patients undergoing craniosynostosis surgery. Pediatric patients aged 2-24 months were divided into a control group (n = 28), whose transfusion therapy was managed using intermittent blood gas analysis, and an experimental group (n = 27), whose transfusion therapy was managed using SpHb monitored with Masimo rainbow® sensors connected to a Radical-7® Pulse CO-Oximeter®. In both groups, blood gas analysis was performed hourly during the perioperative period; in the SpHb group, when SpHb monitoring indicated a sudden decrease in hemoglobin, blood gas analysis was simultaneously performed.
The researchers calculated the following statistically significant (p < 0.05) results:
| Outcome | Control Group | SpHb Group | P-value |
|---|---|---|---|
|
Length of stay in ICU |
55.43 hours ± 25.34 hours (48 hours median) |
33.48 hours ± 12.25 hours (24 hours median) |
0.001 |
|
Postoperative drainage |
215.54 mL ± 93.1 mL |
136.85 mL ± 62.27 mL |
0.001 |
|
Postoperative red blood cell transfusion |
179.02 mL ± 163.06 mL (145 mL) |
102.69 mL ± 73.87 mL (105 mL) |
0.033 |
|
Postoperative fresh frozen plasma transfusion |
71.96 mL ± 94.95 mL (25 mL) |
28.15 mL ± 64.35 mL (0 mL) |
0.043 |
|
Perioperative crystalloid |
396.79 mL ± 171.16 mL (350 mL) |
462.59 mL ± 158.91 mL (500 mL) |
0.048 |
|
First platelet level in ICU |
270,821 ± 74,474 |
327,185 ± 104,644 |
0.025 |
|
Last lactate level in ICU |
1.47 mmol/L ± 0.64 mmol/L (1.25 mmol/L) |
1.18 mmol/L ± 0.63 mmol/L (0.9 mmol/L) |
0.044 |
They found that the length of stay in the ICU was statistically significantly higher in the control group than the SpHb group. Postoperative drainage, red blood cell transfusion, and fresh frozen plasma transfusion in the ICU were also statistically significantly higher in the control group than the SpHb group. Lactate levels were higher in the SpHb group at the start of the operation, but higher in the control group at the end.
The researchers concluded, "Noninvasive continuous hemoglobin monitoring in major hemorrhagic surgeries in pediatric patients might be effective in reducing morbidity not only by reducing the amount of transfusion but also [by] leading to less metabolic and hemodynamic instability."
In other clinical studies, conducted with adult patients, continuous monitoring with SpHb as part of patient blood management (PBM) programs has been found to improve outcomes, such as reducing the percentage of patients receiving transfusions,2 reducing the units of red blood cells transfused per patient,3-4 reducing the time to transfusion,5 reducing costs,6 and even reducing mortality 30 and 90 days after surgery by 33% and 29%, respectively.7 The evidence of SpHb’s impact on outcomes spans the globe, representing 6 countries on 4 different continents.2-8 Today, SpHb technology supports clinicians in over 75 countries around the world.9
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.10 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,11 improve CCHD screening in newborns,12 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.13-16 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,17 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.18 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Saraçoğlu A , Orhon Ergün M, Sakar M, Uyar E, Saçak B, Aykaç Z. The use of SpHb in pediatric patients undergoing major surgery associated with reduced morbidity. Proceedings from the Euroanaesthesia 2020 Annual Meeting. #5291.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Merolle L, Marraccini C, Di Bartolomeo E, Montella M, Pertinhez T, Baricchi R, Bonini A. Postoperative patient blood management: transfusion appropriateness in cancer patients. Blood Transfus 2020; 18: 359-65 DOI 10.2450/2020.0048-20.
- Masimo data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo's COVID-19 Response Efforts Highlighted at California Life Sciences Association's Pantheon 2020
Masimo, the Only Patient Monitoring Company Included, Was One of Seven Featured California Innovators
Irvine, California– December 7, 2020 – Masimo (NASDAQ: MASI) is proud to have been one of seven California innovators featured at Pantheon 2020, the annual event at which the California Life Sciences Association (CLSA) brings together industry leaders in the life sciences to recognize outstanding achievements. Held virtually this year, Pantheon 2020, which took place last Thursday, was anchored by a series of videos and panels highlighting stories of scientific innovation, collaboration, and progress focused on celebrating efforts to combat COVID-19. Masimo, the only patient monitoring company featured, was also one of the three companies highlighted at CLSA's "Celebrating California Innovation" event, and at a session dedicated to exploring how Masimo SafetyNet™ is playing a key role in Masimo's efforts to help clinicians and hospitals around the world fight the pandemic.
Mike Guerra, President and CEO of CLSA, commented, "Masimo is an exemplary illustration of the resiliency and flexibility shown by life sciences companies when California and the world were faced with a global pandemic. The adaptability of their remote patient monitoring technology has saved lives and allowed patients to recover from their homes, thereby freeing up critically needed hospital beds."
In early 2020, harnessing its expertise in noninvasive patient monitoring, automation, and telehealth solutions, Masimo developed Masimo SafetyNet, a secure, home-based patient management platform combining hospital-grade tetherless pulse oximetry, continuous temperature measurement, and a remote data capture and surveillance platform. Designed to help hospitals accommodate surges in COVID-19 patient volume and help lower-acuity patients recover at home and in other alternate care spaces, Masimo SafetyNet allows clinicians to keep an eye on their patients' physiological status – including oxygen saturation, respiration rate, pulse rate, and temperature – from afar, at all times. Already in use at numerous institutions in the U.S. and beyond, Masimo SafetyNet can be rapidly deployed and easily scaled as needed, without additional hardware or network infrastructure, helping caregivers provide the best possible care as the pandemic worsens.
In his speech at Pantheon 2020, Joe Kiani, Founder and CEO of Masimo, said, "I want to thank CLSA for highlighting our work in helping with the global pandemic. I also want to take this time to say we're humbled by this, because in fact, the real heroes are the people on the front lines using the tools we create to help patients, especially those with COVID-19. We've never seen anything like COVID-19, but as a member of CLSA, we're proud to have used life sciences technology, mathematics, and engineering to do the work that we have; together we are making a difference in so many people’s lives."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Study Comparing Two Noninvasive Indicators of Fluid Responsiveness on Mechanically Ventilated Patients Finds Masimo PVi® Effective and Advantageous
Researchers Preferred PVi for Being “Continuous, Operator-independent, and More Reliable”
Neuchatel, Switzerland – November 30, 2020 – Masimo (NASDAQ: MASI) announced today the findings of a study published in the Ain-Shams Journal of Anesthesiology in which Dr. Diaaeldin Aboelnile and colleagues at Ain-Shams University in Cairo, Egypt compared two noninvasive methods of assessing fluid responsiveness in mechanically ventilated patients, dIVC and Masimo PVi®.
They found both methods to be “effective,” but determined that PVi was advantageous because of being “continuous, operator-independent, and more reliable than dIVC.”1 PVi, pleth variability index, is a measure of the dynamic changes in perfusion index that occur during the respiratory cycle.
Noting the importance of predicting responsiveness prior to fluid administration and the drawbacks of invasive and static methods of assessing responsiveness, the researchers sought to assess the effectiveness and reliability of PVi—which is noninvasive, dynamic, continuous, and can be measured using a pulse oximetery sensor—by comparing it to another noninvasive, but non-continuous, static method, ultrasound calculation of inferior vena cava distensibility index (dIVC). dIVC represents the percentage of variation in the inferior vena cava diameter during inspiration versus expiration. To that end, they monitored 88 adult, sedated, mechanically ventilated, intubated surgical patients using both PVi and dIVC. The patients were classified as fluid responders (48) or non-responders (40) by using the passive leg raising (PLR) test; patients whose cardiac index (CI) increased by 15% or more were considered responders. PVi was monitored using a fingertip sensor and a Masimo Radical-7® Pulse CO-Oximeter®; dIVC was measured using a Mindray M5 ultrasound probe. To assess their performance against an invasive method, the researchers also measured central venous pressure (CVP).
The researchers calculated the following results for the three parameters:
| Parameter | CVP | PVi | dIVC |
| Threshold value | ≥ 5 mmHg | > 14% | > 19.42% |
|---|---|---|---|
| Sensitivity | 70.83% | 93.75% | 79.17% |
| Specificity | 47.5% | 87.5% | 80% |
| Area under the curve (95% confidence interval) | 0.612 (0.502-0.714) | 0.955 (0.889-0.988) | 0.886 (0.801-0.944) |
| P-value | 0.0648 ("not significant") | < 0.0001 ("highly significant") | < 0.0001 ("highly significant") |
Based on these findings, the researchers concluded, “The results of our study showed that assessment of PVi and dIVC noninvasively were good predictors for fluid management and responsiveness prediction using PLR technique in the surgical ICU mechanically ventilated patients.” They continued, “PVi and dIVC can be used in the assessment of fluid responsiveness of the intubated ventilated sedated patients with sinus rhythm in the ICU, and both methods are noninvasive and can be performed at the bedside, but PVi has the advantage of being continuous, operator-independent, and more reliable than dIVC.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™;), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™; platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™;, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Aboelnile D, Elseidy M, Kenawey Y, Elsherif I. Prediction of fluid responsiveness in mechanically ventilated patients in surgical intensive care unit by pleth variability index and inferior vena cava diameter. Ain-Shams J Anesth. 2020. 12:48. https://doi.org/10.1186/s42077-020-00097-4.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Study Investigates Effects of Patient Blood Management Program with Masimo SpHb®, Noninvasive, Continuous Hemoglobin Monitoring, on Postoperative Cancer Patients
Patient Blood Management with Masimo SpHb Increased Transfusion Appropriateness and Decreased RBC Units Transfused per Patient
Neuchatel, Switzerland November 16, 2020 - Masimo (NASDAQ: MASI) announced today the findings of a study published in Blood Transfusion in which Dr. Lucia Merolle and colleagues at the Azienda USL-IRCCS of Reggio Emilia, Italy investigated the impact of applying a patient blood management program, including use of noninvasive and continuous hemoglobin monitoring, Masimo SpHb®, to the care of postoperative cancer patients.1 The study found that using SpHb as part of a patient blood management program not only increased how often postoperative blood transfusions were appropriate, but decreased the total and mean number of blood units transfused per patient.
|
Masimo Radical-7® with SpHb® |
Patient blood management (PBM) is "an evidence-based, multidisciplinary approach aimed at optimizing the care of patients who might need blood transfusions." Recognizing that PBM might have specific benefits for surgical oncology patients, the researchers implemented a two-step PBM program and compared three groups of postoperative adult cancer patients who underwent major surgery between 2014 and 2017. Step 1 PBM included seminars and training designed to teach semi-intensive post-surgical personnel the principles of PBM. Step 2 PBM added the use of SpHb monitored with Masimo Radical-7® Pulse CO-Oximeters® with SpHb. Audit 1 reviewed data for 200 patients whose post-surgical care did not incorporate PBM. Audit 2 was of 200 patients whose care incorporated Stage 1 PBM, and Audit 3 was of 200 patients whose care incorporated Stage 2 PBM along with continuous SpHb monitoring.
Using guidelines developed by the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI), the researchers found that transfusion appropriateness rose from 38% in Audit 1 patients, to 75% in Audit 2 patients (Step 1 PBM), to 79% in Audit 3 patients (Step 2 PBM, with SpHb). The total number of red blood cell (RBC) units transfused was similar for Audit 1 and Audit 2 patients (52 and 58 units, respectively), but dropped to 39 units with the addition of SpHb monitoring to PBM (Audit 3). The mean number of RBC units transfused was the same for Audit 1 and Audit 2 patients (1.8 units/patient), but again, with the addition of SpHb monitoring (Audit 3), the mean dropped to 1.3 units/patient.
The researchers concluded, "Our PBM bundle positively impacted RBC transfusion appropriateness in post-surgical cancer patients, both in terms of quality and quantity. A structured PBM program specifically dedicated to surgical oncology should cover the entire perioperative period and might further improve transfusion appropriateness in these patients. The publication of guidelines on the management of anemia in surgical oncology should be a priority."
In other clinical studies, continuous monitoring with SpHb as part of PBM programs has been found to improve outcomes, such as reducing the percentage of patients receiving transfusions,2 reducing the units of red blood cells transfused per patient,3-4 reducing the time to transfusion,5 reducing costs,6 and even reducing mortality 30 and 90 days after surgery by 33% and 29%, respectively.7 With the addition of the Italian study, the evidence of SpHb's impact on outcomes spans the globe, representing 6 countries on 4 different continents.1-7 Today, SpHb technology supports clinicians in over 75 countries around the world.8
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.9 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,10 improve CCHD screening in newborns,11 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.12-15 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,16 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.17 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Merolle L, Marraccini C, Di Bartolomeo E, Montella M, Pertinhez T, Baricchi R, Bonini A. Postoperative patient blood management: transfusion appropriateness in cancer patients. Blood Transfus 2020; 18: 359-65 DOI 10.2450/2020.0048-20.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Masimo data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Founder and CEO and Co-Inventor of Measure-Through-Motion Pulse Oximetry, Joe Kiani, Awarded by the Ibero-American Society of Neonatology for Improvements to Neonatal Health in Latin America
Irvine, California - November 9, 2020 - Masimo (NASDAQ: MASI) announced today that its Founder, Chairman, and CEO, Joe Kiani, has been honored by the Ibero-American Society of Neonatology (SIBEN) at its 17th Annual Congress (this year held virtually) with the Award for Improvement of Neonatal Health in Latin America. In her award presentation, SIBEN Scientific Group member Dr. Susana Rodríguez praised Mr. Kiani for his lifelong dedication to improving newborn care.
|
Joe Kiani |
SIBEN, founded in 2004, is dedicated to improving newborn care throughout Latin America – where 15 babies die every hour, with 60% of those who die before their first birthday dying during the first 28 days after birth.1 SIBEN is comprised of more than 2,000 healthcare professionals associated with neonatal care.
Dr. Rodríguez noted that Mr. Kiani's commitment to neonatal care started with the invention, in 1995, of Masimo Signal Extraction Technology®, or SET®, pulse oximetry, whose breakthrough ability to measure through motion and low perfusion led to remarkable improvements in neonatal care. In particular, use of SET® has played a key role in reducing the rate of neonatal blindness (retinopathy of prematurity). In 2003, Dr. Augusto Sola (Managing Director of SIBEN) and colleagues showed that using a new protocol with Masimo SET®, clinicians reduced ROP to nearly zero over five years.2 A later study showed that the protocol's success depended on SET® technology, as the same protocol with a competing pulse oximeter did not reduce ROP.3 Today, SET® pulse oximetry is used to monitor more than 200 millions patients each year,4 and has been shown, among other benefits, to help improve critical congenital heart disease (CCHD) screening in newborns.5
Continuing, Dr. Rodríguez highlighted how Mr. Kiani's creativity and vision have led to many additional medical advancements in the years since, with Masimo, which recently celebrated its 31st anniversary, remaining at the forefront of global medical innovation. Mr. Kiani has long championed the importance of patient safety, and in 2013 created The Patient Safety Movement Foundation, dedicated to achieving zero preventable deaths by 2030. To date, more than 4,700 hospitals in 50 countries have committed to this goal—and an estimated 93,000 lives are being saved annually.6 Among other honors and awards, Mr. Kiani has been named SafeCare’s Person of the Year and one of "50 Experts in Patient Safety" by Becker’s Hospital Review. He received an Honorary Doctorate of Science from Chapman University, and is an Honorary member of the Mexican Academy of Surgery.
In his acceptance speech, Mr. Kiani said, "Thank you to all the neonatologists and the care providers around neonates. There’s so much apathy, and I think that's the biggest hurdle to improving patient care – but not with neonatologists and neonatal nurses. You have never stopped amazing me with how much you care for our babies, your patients. I also want to thank SIBEN for the incredible work you're doing to bridge the gap between knowledge and what's practiced. You know better than I that what’s common practice is unfortunately not always best practice – and your work – to educate, educate, educate – is critical in bridging that gap, so more babies get the best care possible. I want to thank Dr. Susana Rodríguez and the board of SIBEN for honoring me with this meaningful award."
Kiani continued, "I want to tell you how blessed I feel to have friends like Mohammad Diab and Dr. Augusto Sola, who helped me with a great innovation, not only to innovate our measure-through-motion pulse oximetry, but to innovate a way to use it to help so many babies. That gift, which these two incredible friends have given me, has allowed me to feel fulfilled, and even though I know I'm just a speck in time and in space, at least we’ve been together, my dear friends, a useful speck, that may have given birth and happiness to more specks to enjoy the joy and pain of life, which is precious. Thank you again for this incredible award. Thanks for encouraging me to try harder, and for encouraging others to try harder, to do the right thing."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.7 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.8-11 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,4 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- SIBEN data on file.
- Chow LC, Wright KW, Sola A. Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants? Pediatrics. 2003 Feb;111(2):339-45.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Masimo data on file.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Patient Safety Movement Foundation data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces Limited Market Release of Radius VSM™
Versatile, Wearable Continuous Monitoring Solution Provides Masimo SET® Pulse Oximetry, Proprietary Noninvasive Blood Pressure, Body Temperature, Respiration Rate, and Electrocardiography
NEUCHATEL, SWITZERLAND - November 2 2020 - Masimo (NASDAQ: MASI) announced today that Radius VSM™, a wearable, tetherless vital signs monitor, has received CE marking and is being released in limited European markets. The versatile, expandable Radius VSM provides the ability to monitor a wide variety of physiological measurements, including continuous SET® pulse oximetry, noninvasive blood pressure, body temperature, respiration rate, and electrocardiography (ECG). Designed on a wearable, modular platform, Radius VSM features can be scaled to accommodate surges in patient volume and for use across the continuum of patient care, based upon each patient’s needs and level of acuity. For additional versatility, Radius VSM can operate as a self-contained device or be used wirelessly with Masimo bedside monitors and patient surveillance systems – automating the integration of expanded monitoring and the transfer of continuous monitoring data to electronic medical records (EMRs)
|
Masimo Radius VSM™ |
As a comprehensive, scalable solution, Radius VSM has potential clinical utility in a multitude of care scenarios while also encouraging ambulation and freedom of movement, which studies have shown to be a key factor in more rapid patient recovery.1-2 Configurable with a variety of modules and noninvasive sensors designed to prioritize comfort and ergonomics, care teams can seamlessly increase or decrease the technologies monitored based on each patient's needs, without additional bedside equipment, infrastructure, or tethered connections. Radius VSM offers the following wearable technologies:
- • Clinically proven Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry,3 including oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), PVi® fluid responsiveness, and RRp® plethysmographic respiration rate
- • ECG with heart rate, respiration rate, and advanced lethal arrhythmia detection using 3-leadwire single-patient-use electrodes offering 6 ECG waveforms: I, II, III, aVR, aVL, and aVF
- • Measure-on-inflation noninvasive blood pressure that features single-patient-use cuffs, customizable scheduling (eliminating the need for periodic manual clinician measurement), and variable inflation speeds; for example, quicker for ambulating patients and slower for resting patients, minimizing the possibility of sleep disruption
- • Continuous body temperature measurements with notifications when clinician-specified temperature thresholds are breached
- • RRa® continuous acoustic respiration rate monitoring using rainbow Acoustic Monitoring®, which converts the acoustic patterns caused by the patient's airflow into breath cycles to calculate respiration rate and visualize an acoustic respiration waveform
Radius VSM features a high-resolution touchscreen and the ability to store and display up to four hours of trend and waveform data on the device itself, providing additional clinical context at the point of care for nurses, therapists, and doctors when interacting directly with patients. In addition, Radius VSM's rechargeable battery lasts over twelve hours between charges; two devices are supplied for each bedside to provide uninterrupted functionality. Radius VSM has a rugged, durable exterior designed to withstand busy hospital environments and drops of up to one meter, and is water resistant.
Radius VSM can communicate via Bluetooth® to the Root® Patient Monitoring and Connectivity Hub at the bedside, for enhanced visibility on Root’s display. The device can also independently communicate via Wi-Fi to the Patient SafetyNet™ Supplemental Remote Monitoring and Clinician Notification system, enabling use as a patient surveillance system monitored from central viewing stations. The ability to track continuous vital signs and telemetry data from afar is an especially relevant feature for clinicians in the context of COVID-19 and other contagious conditions, where staying informed of patient status while minimizing the risk of cross-contamination can be essential to effective patient care. In addition, automated remote data transfer from Radius VSM to electronic medical records (EMRs) ensures that up-to-date physiological data is always available to clinicians and systems throughout the hospital, without the need for time-consuming manual transcription.
Joe Kiani, Founder and CEO of Masimo, said, "Radius VSM offers the reliability and accuracy of a bedside monitor with the flexibility of a wearable device, thus opening the door to numerous new uses, made possible by its unique scalability and broad range of automated, continuous measurements. We’re excited for clinicians and patients in Europe to experience the advantages of Radius VSM, and look forward to making it available to the rest of the world as regulatory clearances are obtained."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,10 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.11 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Needham D et al. Archives of Physical Medicine and Rehabilitation. Vol 91, Issue 4, PP 536–542, April 2010.
- Ronnenbaum J et al. J Acute Care Phys Ther. 2012;3(2):204-210.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Radius VSM™, SET®, and RRa®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Radius VSM, SET®, and RRa, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Dr. Augusto Sola, Masimo VP of Medical Affairs for Neonatology, Honored by the American Academy of Pediatrics with Pioneer Award
Dr. Sola Pioneered New Protocol Using Masimo SET® Pulse Oximetry That Dramatically Reduced Blindness and Eye Damage in Neonates
IRVINE, CALIFORNIA – October 19, 2020 - Masimo (NASDAQ: MASI) announced today that Augusto Sola, MD, Vice President of Medical Affairs for Neonatology at Masimo, has been awarded the 2020 Pioneer Award, Section of Neonatal Perinatal Medicine, by the American Academy of Pediatrics (AAP). The honor recognizes the groundbreaking achievements and contributions Dr. Sola has made, using his Masimo SET®-based protocol, to improve the health and well-being of newborn infants.
|
Dr. Augusto Sola |
Dr. Sola's impressive career in neonatology has improved the lives of countless newborns in the U.S., Latin America, and across the world. Dr. Sola's innovative research on oxygen administration and monitoring oxygen saturation in preterm infants has played a key role in reducing the rate of neonatal blindness (retinopathy of prematurity) and our understanding of the impact of various neonatal practices on the developing brain. Dr. Sola has published 130 original articles in peer-reviewed journals, 390 review articles, and 5 neonatology textbooks, as well as delivered more than 3,500 lectures to research and clinical groups around the world. Dr. Sola also founded the Ibero-American Society of Neonatology (SIBEN), dedicated to continuous quality improvement in neonatal care throughout the Americas.
Dr. Sola received his MD at Buenos Aires University School of Medicine and completed his Pediatric Residency and Chief Pediatric Residency at the University of Massachusetts, followed by a Neonatal Fellowship at the University of California, San Francisco. In neonatal practice since 1974, Dr. Sola has been Professor of Pediatrics at Buenos Aires University Medical School, the University of California, San Francisco, the University of California, Los Angeles, and Emory University. In addition to his position at Masimo, Dr. Sola continues to work directly with critically ill newborns.
Dr. Sola’s seminal work was done in 1998 at Cedars-Sinai Medical Center in Los Angeles. The results, published in 2003 by Drs. Sola, Wright, and Chow, showed that using a new protocol with Masimo SET®, clinicians reduced ROP to nearly zero over five years.1 Dr. Sola and colleagues later showed at Emory that the protocol's success depended on SET® technology, as the same protocol with a competing pulse oximeter did not reduce ROP.2 Dr. Sola’s work on the reduction of ROP through oxygen saturation targeting has now become the standard of care.3-5
Dr. Sergio Golombek, MD, MPH, Professor of Pediatrics at New York Medical College, Neonatologist, Ex-President of SIBEN, and AAP member, commented, "In 1952, a Chicago newspaper wrote: 'The best friend a baby ever had,' referring to pediatrician Isaac A. Abt, MD, FAAP (1867-1955), founder of the AAP and its first president in 1930. He was known as a leading clinician, academic, advocate, promoter, writer, and leader. I think this has been overcome by Dr. Sola, who is, in my opinion, the best friend of a baby and his or her parents and of the many neonatal professionals and trainees whom he trained!"
Joe Kiani, Founder and CEO of Masimo, said, "We are incredibly proud of Dr. Sola, a brilliant and caring doctor whom I have had the privilege of knowing for many years. Dr. Sola's pioneering work on the oxygen saturation of neonates has made a significant impact on the prevention of blindness in infants, and his contributions to our understanding of neonatal physiology are vast. It is only fitting that the American Academy of Pediatrics has chosen to honor him. We are grateful to benefit from his expertise at Masimo, which has been especially dedicated, ever since its founding, to improving outcomes for the youngest and most fragile patients. Congratulations, Dr. Sola, on this well-deserved recognition."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.6 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,7 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.8-11 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,12 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.13 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Chow LC, Wright KW, Sola A. Can Changes in Clinical Practice Decrease the Incidence of Severe Retinopathy of Prematurity in Very Low Birth Weight Infants? Pediatrics. 2003 Feb;111(2):339-45.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Bizzarro MJ et al. Temporal Quantification of Oxygen Saturation Ranges: An Effort to Reduce Hyperoxia in the Neonatal Intensive Care Unit. J Perinatol. 2014 Jan;34(1):33-8
- Baquero H et al. Avoiding Hyperoxemia during Neonatal Resuscitation: Time to Response of Different SpO2 Monitors. Acta Paediatr. 2011 Apr;100(4):515-8.
- Sola A et al. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014 Oct;103(10):1009-18. doi: 10.1111/apa.12692.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Researchers Use Masimo ORi™, Oxygen Reserve Index, to Predict the Need for Postoperative Oxygen Therapy in Pediatric Patients with Obstructive Sleep Apnea
Neuchatel, Switzerland – October 12, 2020 - Masimo (NASDAQ: MASI) today announced the findings of an abstract recently presented at ANESTHESIOLOGY® 2020, the Annual Meeting of the American Society of Anesthesiologists (ASA). In this independent, retrospective study, researchers investigated whether Masimo ORi™ (Oxygen Reserve Index) could predict whether children with obstructive sleep apnea syndrome (OSAS) undergoing tonsillectomy required postoperative oxygen therapy.
|
Masimo Root® with ORi™ |
ORi, available outside the U.S., is a noninvasive and continuous parameter intended to provide insight into a patient’s oxygen status during moderate hyperoxia. Enabled by the multi-wavelength rainbow® Pulse CO-Oximetry platform, ORi is provided alongside oxygen saturation (SpO2), a clinically proven Masimo SET® pulse oximetry measurement.
Dr. Yoshimi Inagaki and colleagues at Tottori University in Yonago, Japan sought to determine whether Masimo ORi could serve as a useful predictor of the need for postoperative oxygen therapy (POT). They enrolled 45 pediatric patients with OSAS, ranging from 7 to 120 months, who were anesthetized with sevoflurane and monitored with ORi while undergoing tonsillectomy.
Of the 45 patients, 16 required POT. For those 16, the mean lowest ORi and SpO2 values were 0.28 and 93%, respectively. For the remaining patients, who did not receive POT, the mean lowest values were 0.64 and 97%, respectively. The researchers calculated sensitivity and specificity for ORi predicting when POT would not be needed of 0.815 (95% confidence interval 0.5435 – 0.9595) and 0.9310 (95% confidence interval 0.7723 – 0.9915), respectively.
The researchers concluded that ORi is "likely to become an index of POT in pediatric patients with OSAS." They also noted, "In children with OSAS, [the] requirement of POT following tonsillectomy and adenoidectomy is probably able to be predicted on the basis of the results of this retrospective cohort study."
ORi has not received FDA 510(k) clearance and is not available for sale in the United States.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Inagaki Y, Kitagawa Y, Otsuki A. Oxygen Reserve Index Predicts Postoperative Oxygen Requirement after Tonsillectomy in Children with Obstructive Sleep Apnea Syndrome. Proceedings from the ASA 2020 Annual Meeting. Abstract #A4091. Accessed 10/8/20 at https://epostersonline.com/asa2020/node/739?view=true.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo ORi™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo ORi, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces Consumer Launch of the Radius T°™ Continuous Thermometer, a More Convenient Way to Keep Track of Fever
Wearable, Wireless Sensor Provides Hassle-Free Continuous Body Temperature Measurements
Irvine, California – October 5, 2020 - Masimo (NASDAQ: MASI) today announced the launch of the Radius T°™ Continuous Thermometer for consumers. Unlike spot-check, episodic thermometers, the wearable, wireless Radius T° measures body temperature continuously, seamlessly transmitting data and customizable temperature notifications to the user's smartphone – helping caregivers, such as parents, monitor loved ones' temperatures even while they sleep.
|
Masimo Radius T°™ Continuous Thermometer |
Radius T° represents a paradigm shift in thermometry by making it continuous, wearable, and hassle-free. Traditional periodic and invasive methods depend on the user repeatedly conducting a series of steps that can interrupt daily activities, including sleep, and can miss body temperature trends and patterns. With a traditional thermometer, a person may only notice a spike in temperature hours after a spike has occurred, or may not even become aware of it if it is during sleep. By contrast, Radius T° continuously and seamlessly measures temperatures using a small, inconspicuous, wearable sensor that can be easily applied to anyone from children to elderly adults – with no action needed after initial application to the skin. Radius T° eliminates manual measurements while providing continuous insight into changes in the user's temperature and helps users understand which way their temperature is trending. In addition, Radius T° uses proprietary algorithms to provide body temperature measurements for users five years or older that approximate oral temperature, not just external skin temperature. Radius T° provides temperature measurements with laboratory accuracy within ±0.1°C, whereas other oral thermometry solutions typically have laboratory accuracy within ±0.2°C.
Earlier this year, Masimo launched Radius T° as part of the Masimo SafetyNet™ remote patient management solution, for use both in hospitals and by patients at home. Dr. Neal Fleming, M.D., Ph.D., Vice Chair for Education in the Department of Anesthesiology and Pain Medicine at UC Davis Health, commenting on his experience using Radius T°, said, "Radius T° is noninvasive and convenient for patients. I do not have to interrupt their daily activities or their sleep and it provides me continuous trend data that is a powerful guide to patient care. It makes it easier for me to recognize possible changes in their symptoms."
Flexible and slim, each disposable Radius T° sensor can be worn comfortably for up to eight days, and is water resistant during shower and exercise. Users are free to carry on with their daily activities and sleep, without interruption or hassle – all while Radius T° continuously collects temperature data. Using built-in Bluetooth®, the sensor easily pairs with the Masimo Radius T° App on the user’s smartphone, providing real-time temperature values with user-definable automatic notifications (for example, when temperature exceeds a certain user-selected threshold or if it spikes), as well as detailed historical trending data, revealing the baseline and fluctuation patterns unique to each person that can help users determine whether a rise in temperature warrants action. In addition, the user-friendly Masimo Radius T° App can support multiple family member profiles and can be easily set to schedule medication reminders.
Joe Kiani, Founder and CEO of Masimo, said, "We're excited to expand our growing line of consumer solutions, which includes MightySat® and Masimo Sleep™, with the Radius T° Continuous Thermometer. For years, clinicians have trusted Masimo technology to monitor patients in the hospital. With Radius T° and our other consumer solutions, we're bringing our expertise and experience in accurately and reliably measuring physiological data from the hospital to the home."
Radius T° is not FDA 510(k) cleared. The device is marketed under the FDA's Enforcement Policy for Clinical Electronic Thermometers During COVID-19. Radius T° is CE marked.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Radius T°™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Radius T°, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Announces FDA Clearance of the Rad-G™ Pulse Oximeter
Rugged Handheld Device Provides Clinically Proven Masimo SET® Pulse Oximetry and Respiration Rate Monitoring
Irvine, California – September 28, 2020 - Masimo (NASDAQ: MASI) today FDA clearance of the Rad-G™ Pulse Oximeter, a rugged handheld device that provides clinically proven SET® pulse oximetry, respiration rate from the pleth (RRp®), and other vital parameters for both spot-checking and continuous monitoring. With its long-lasting rechargeable battery, robust rubber casing, light weight, and a convenient new direct-connect sensor capable of monitoring both adults and children, Rad-G makes it easier for clinicians to quickly assess patients and make informed care decisions anywhere pulse oximetry or vital signs checking is needed in a compact, portable form factor. Providing the ultimate in handheld versatility, Rad-G can be used in a variety of settings, including but not limited to physicians' offices, outpatient services, long-term care facilities, wellness clinics, first-response scenarios, and limited-resource environments.
|
Masimo Rad-G™ Pulse Oximeter |
Launching alongside the device, the new multipurpose, direct-connect Rad-G Sensor is indicated for monitoring both adult and pediatric patients. By eliminating the need to stock and carry multiple sensor types, the Rad-G Sensor further increases Rad-G's versatility and ease of use, especially in more challenging field environments. In addition to this innovative new sensor, Rad-G is compatible with the vast portfolio of Masimo reusable and single-patient-use sensors, maximizing its flexibility and offering clinicians the ability to customize the solution based on the unique needs of each care setting.
First developed in partnership with The Bill & Melinda Gates Foundation as a spot-check device for global use, the 2021 Rad-G expands on its predecessor's capabilities with the addition of alarms and thus the ability to provide continuous monitoring – without sacrificing any portability, convenience, or ruggedness. Rad-G's rechargeable battery provides an impressive 24 hours of use between charges, allowing clinicians to work in outdoor, transport, and emergency scenarios with confidence that Rad-G will continue to function hour after hour. All the while, the high-resolution screen displays a continuous pleth waveform and its fully configurable, audible alarms help alert clinicians to changes in patient status that may require their intervention.
In addition to Masimo SET® oxygen saturation (SpO2), pulse rate (PR), perfusion index (Pi), and pleth variability index (PVi®), Rad-G is also notable for its ability to use the same sensor to monitor respiration rate from the plethysmograph, with RRp. With breathing difficulty generally considered one of the earliest signs of patient deterioration, Masimo hopes that the availability of RRp on Rad-G may play a role in assisting clinicians and public health officials as they seek to combat respiratory-related illnesses, including pneumonia and COVID-19.
SpO2 and PR monitoring on Rad-G is provided using clinically proven Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other technologies.1 SET® is estimated to be used on more than 200 million patients a year2 and is the primary pulse oximetry at 9 of the 10 hospitals that top the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.3 With Masimo SET® technology in Rad-G, clinicians have access to hospital-grade, accurate pulse oximetry measurements in the palm of their hands.
Joe Kiani, Founder and CEO of Masimo, said, "With the Rad-G Pulse Oximeter, we set out to create an accessible, high-quality care solution that clinicians can rely on in a multitude of care settings. We are bringing our expertise in pulse oximetry to a smaller, more lightweight, rugged, and versatile handheld device – without sacrificing any of the advantages that help provide clinicians with critical insights into patient status. As the COVID-19 pandemic continues, it's more imperative than ever that caregivers have access to the most accurate and reliable pulse oximetry monitoring technologies. We are proud to offer Rad-G among our suite of solutions powered by the breakthrough technology, SET® pulse oximetry."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-9 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,2 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.3 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Rad-G™, SET®, and RRp®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Rad-G, SET®, and RRp, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Modern Healthcare Honors Dr. Peter Pronovost as a Top 25 Innovator for Spearheading Remote Monitoring Program at University Hospitals Using Masimo’s Technology
|
Dr. Peter Pronovost with Masimo SafetyNet™ |
Irvine, California – September 2, 2020 - Masimo (NASDAQ: MASI) today announced that Peter J. Pronovost, MD, PhD, Chief Quality and Clinical Transformation Officer at University Hospitals in Cleveland (UH), has been named a "Top 25 Innovator" by Modern Healthcare for his contribution to University Hospitals’ launch of the Masimo SafetyNet™ remote patient management solution for people who tested positive for COVID-19.
Long recognized throughout the industry for his expertise and innovation in patient safety and quality of care, Pronovost led a team earlier this year to pilot Masimo SafetyNet, a secure telehealth solution that combines tetherless Masimo SET® pulse oximetry with a remote patient surveillance platform programmed with a COVID-19 care protocol. "Masimo SafetyNet was transformational in helping us respond to the pandemic by providing UH an innovative, high-value way to help us prepare for a potential surge in patient volume," said Pronovost.
Since its initial deployment in March, Masimo SafetyNet has expanded to allow continuous temperature measurement using the tetherless Radius T°™ sensor. Masimo SafetyNet is now in use at more than 130 institutions around the world.
Joe Kiani, Founder and CEO of Masimo, commented, "Dr. Pronovost, with his pioneering work on Masimo SafetyNet, has helped pave the way to save the lives of COVID-19 patients – a testament to his vision and dedication. On behalf of the many thousands who have benefited and who will benefit from his passion and brilliance over the span of his career, I am thrilled to congratulate him on this well-deserved honor. His values align closely with Masimo’s commitment to improving patient outcomes and reducing the cost of care."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo O3® Regional Oximetry Receives FDA Clearance for Somatic Applications and Measurement of Relative Changes in Cerebral Hemoglobin
Irvine, California – August 31, 2020 - Masimo (NASDAQ: MASI) today announced that O3® Regional Oximetry has received FDA clearance for expanded use in monitoring somatic tissue oxygenation saturation in all patient populations and monitoring relative changes in hemoglobin, oxyhemoglobin, and deoxyhemoglobin in adult brains. With this FDA clearance, O3 is now indicated for use in both cerebral and somatic applications, both in the U.S. and in CE mark countries, for all patient populations.
|
Masimo Root® with O3® Regional Oximetry |
O3 Regional Oximetry provides regional or tissue hemoglobin oxygen saturation with a trending specification of 3% ARMS* (cerebral and somatic, all ages) and absolute accuracy specifications of 4% ARMS (cerebral, adults) and 5% ARMS (cerebral, pediatric patients) through the use of O3 multi-wavelength sensors and O3 Regional Oximetry near-infrared spectroscopy (NIRS) technology. Unlike peripheral pulse oximetry, which reflects the body's general arterial blood oxygenation, O3 provides information about the local tissue’s hemoglobin oxygen saturation, both in cerebral and somatic applications. This information provides additional insight that may help inform clinicians of changes in cerebral or somatic tissue oxygen levels. Monitoring renal tissue oxygenation in neonates has been found to help provide early warning of renal dysfunction.1 Monitoring both brain and somatic tissue oxygenation simultaneously may further improve clinicians' ability to provide rapid and accurate care.2
André Denault, MD, PhD, Department of Anesthesiology, Critical Care Program at the Montreal Heart Institute and Central Hospital of the University of Montreal, said, "There is a growing interest in the use of somatic NIRS owing to the association of cerebral and somatic desaturation with unfavorable outcomes in shock states. As an addition to O3 cerebral oximetry, the somatic component could serve as an earlier warning of impaired tissue perfusion. Somatic NIRS has been validated as a monitor of peripheral perfusion and shows an excellent correlation with peripheral perfusion compared with radionuclide plethysmography. As previously reported,3 cerebral and somatic NIRS combined with bedside whole-body ultrasound can help in early detection of different types of shock to formulate proper therapeutic strategies."
Along with FDA clearance for somatic monitoring, the O3 measurements ΔcHb, ΔO2Hb, and ΔHHb are cleared for the monitoring of relative changes in oxygenated hemoglobin (ΔO2Hb), deoxygenated hemoglobin (ΔHHb), and total hemoglobin (ΔcHb) in adult brains. With the expanded indications for ΔcHb, ΔO2Hb, and ΔHHb, clinicians gain information that may provide insight into the dynamic relationship between oxygen and hemoglobin in the brain that brain oxygenation saturation (rSO2) alone may not provide.
Dr. Aamer Ahmed, FRA FESC FACC, Consultant Cardiovascular Anesthesiologist at University Hospitals of Leicester, UK, said, "When using regional oximetry to monitor the brain, rSO2 helps track the oxygenation state of the brain, but understanding the dynamic variations in rSO2 is even more valuable. Changes in rSO2 may be a function of a change in hemoglobin or perfusion, or may result from an oxygen desaturation event. In my practice, I use Masimo O3 ΔcHb, ΔO2Hb, and ΔHHb to gain insight into the relative changes in hemoglobin and perfusion in the brain to help enable earlier detection and intervention during adverse changes to cerebral blood flow."
O3 seamlessly integrates with Masimo SedLine® brain function monitoring on the Root® Patient Monitoring and Connectivity Platform, a powerful, expandable hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions. Root's plug-and-play expansion capabilities allow clinicians to simultaneously monitor with O3, SedLine, and other measurements, such as SET® Measure-through Motion and Low Perfusion™ pulse oximetry, providing clinicians with expanded visibility of oxygenation status. Additional modalities available on Root include advanced rainbow® noninvasive measurements such as total hemoglobin (SpHb®) and PVi® (an indicator of fluid responsiveness), NomoLine® capnography, and more – all via an easy-to-interpret, customizable display. Using Root in combination with Masimo Iris Gateway®, monitoring data from O3 can be automatically charted in electronic medical records (EMRs).
Joe Kiani, Founder and CEO of Masimo, said, "O3's expanded indication as a monitor of the oxygenation and deoxygenation components of cerebral tissue, along with the oxygen saturation of somatic tissue, represents an important milestone in helping clinicians and researchers shed additional light on how the body utilizes oxygen and in uncovering organ hypoxemia."
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,5 improve CCHD screening in newborns,6 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.7-10 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,11 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.12 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Hoffman et al. Perioperative perfusion assessed by somatic NIRS predicts postoperative renal dysfunction. Anesthesiology 2005; 103: A1327*.
- Lecluyse et al. Desaturation in the Intensive Care Unit: Preliminary Experience and Review. J Cardiothoracic Vascular Anesth. (2017) 1805-1809.
- Denault A et al. A Practical Approach to Cerebro-Somatic Near-Infrared Spectroscopy and Whole-Body Ultrasound. J Cardio Vasc Anesth. 2019;03.039. DOI: https://doi.org/10.1053/j.jvca.2019.03.039
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo O3®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo O3, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Studies Investigate the Accuracy of Noninvasive, Continuous Hemoglobin Monitoring with Masimo SpHb® on Neonates
Neuchatel, Switzerland – August 19, 2020 - Masimo (NASDAQ: MASI) today announced that in a study recently published in the Journal of Tropical Pediatrics, researchers evaluating Masimo SpHb® (noninvasive, continuous hemoglobin monitoring) on neonatal patients concluded that the technology "offers reliable Hb [hemoglobin] values, which are comparable with the more traditional tHb [invasive venous blood sampling]."1
|
Masimo Root® with SpHb® |
Noting that "iatrogenic anemia caused by diagnostic blood sampling is a common problem in intensive care units," especially in neonatal intensive care units (NICUs), and that diagnostic blood sampling also has implications for infection control and health care costs, Drs. Halil Kazanasmaz and Mahmut Demir at Harran University in Sanliurfa, Turkey sought to assess the efficacy of noninvasive, continuous hemoglobin monitoring with SpHb on neonates by comparing it to conventional tHb. To investigate this, they studied 310 neonatal patients in a level III NICU in Turkey. Immediately after patients had blood drawn for tHb analysis using a hematological laboratory analyzer, their SpHb values (alongside other noninvasive measurements) were recorded using a Masimo Radical-7® Pulse CO-Oximeter® with rainbow® sensors placed on the neonate’s left foot.
Using Bland-Altman analysis, the researchers found a mean bias of 0.05 g/dL between tHb and SpHb, with limits of agreement of -1.85 and 1.96 g/dL. Based on the study results, they concluded, "In this study, it was found that the SpHb measurement method could yield reliable results similar to tHb measurement method in the newborns. It is thought that the SpHb method may have a complementary role in reducing unnecessary blood sampling and therefore iatrogenic anemia, especially in the newborns with anemia. Further clinical studies are needed to define the effectiveness of this measurement method in critically ill patients with circulatory disorders."
In a similar study published earlier this year in the Journal of Clinical Neonatology, Drs. Ahmed Jamal and Biju John at Armed Forces Medical College in Pune, India, compared conventional laboratory hemoglobin to SpHb in 100 hemodynamically stable neonatal patients at a tertiary hospital in southern India.2 To measure SpHb, they similarly used Radical-7 devices with rainbow® sensors, but the sensors were placed on the babies’ wrists instead of the foot. Analyzing the paired Lab-Hb and SpHb data with Bland-Altman analysis, the researchers found “a good visual agreement between the Lab-Hb and SpHb values,” and calculated bias ± precision of 0.763 ± 1.349 g/dL. Drs. Jamal and John similarly concluded that SpHb is "an efficacious method of assessing Hb trend among neonates."
In clinical studies with adult patients, continuous monitoring with SpHb as part of blood management programs has been shown to improve outcomes, such as reducing the percentage of patients receiving transfusions,3 reducing the units of red blood cells transfused per patient,4-5 reducing the time to transfusion,6 reducing costs,7 and even reducing mortality 30 and 90 days after surgery by 33% and 29%, respectively.8 Today, SpHb technology supports clinicians in over 75 countries around the world.9
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
Noninvasive, continuous SpHb for neonatal patients received CE marking in August 2019, and SpHb is now available for patients of all ages in CE marked countries. Noninvasive, continuous SpHb has received FDA clearance for patients > 3 kg but is not currently indicated for patients < 3 kg in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.10 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,11 improve CCHD screening in newborns,12 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.13-16 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,17 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.18 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Kazanasmaz H, Demir M. The Comparison of Hemoglobin Values Measured by Blood and Continuous Non-Invasive Monitoring (SpHb) in Newborn Infants. J Trop. Pediatrics. 2020,0,1o-6. DOI: 10.1093/tropej/fmaa050.
- Jamal A, John B. Efficacy of Noninvasive Hemoglobin Measurement by Pulse CO-Oximetry in Neonates. J Clin Neonatol. 2020;9:57-62.
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014. 5:9. 2.
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4.
- Imaizumi et al. Continuous and noninvasive hemoglobin monitoring may reduce excessive intraoperative RBC transfusion. Proceedings from the 16th World Congress of Anaesthesiologists, Hong Kong. Abstract #PR607.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Masimo data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo PVi® Receives FDA Clearance as an Indicator of Fluid Responsiveness on Mechanically Ventilated Patients
Irvine, California – August 14, 2020 - Masimo (NASDAQ: MASI) today announced that PVi® has received FDA clearance as a continuous, noninvasive, dynamic indicator of fluid responsiveness in select populations of mechanically ventilated adult patients. PVi, or pleth variability index, is a measure of the dynamic changes in perfusion index that occur during the respiratory cycle.
|
Masimo Root® with PVi® and SpHb® |
William C. Wilson, MD, MA, Chief Medical Officer at UCI Health, said, "Anesthesiologists and critical care physicians have long recognized the importance of dynamic measures of intravascular volume and fluid responsiveness. Previously this data could only be acquired using an invasive arterial line, and/or additional sophisticated devices. With the Masimo pleth variability index (PVi), one can now obtain this essential data using the pulse oximeter probe and following the continuous readout on the monitor. The PVi technology has undergone rigorous peer-reviewed evaluation, and demonstrated efficacy in determining adequacy of intravascular volume for guiding goal-directed therapy. The recent clearance of the Masimo PVi system will serve as another major breakthrough in promoting patient safety."
Available alongside Masimo SET® pulse oximetry and rainbow® Pulse CO-Oximetry on a variety of 2-LED, 4-LED, and 8-LED Masimo sensors, PVi is an index between 0 and 100 that is calculated using a proprietary algorithm based upon the relative variability of the pleth waveform.
Hospital protocols such as Enhanced Recovery After Surgery (ERAS) and Goal-directed Therapy (GDT) recommend fluid management as part of larger initiatives designed to improve patient care and safety. Fluid management protocols look to balance fluids by identifying when patients may be fluid responsive. The utility of PVi as a fluid responsiveness indicator has been demonstrated in more than 100 independent, published studies, including in ERAS and GDT protocols.1 For example:
- ERAS: In a study of 109 patients undergoing colorectal surgery, researchers found that the implementation of an ERAS protocol which included PVi led to substantial reductions in lengths of stay (from a median of 5 days to 3 days), complication rates (from 30.1% to 14.7%), and costs per patient (from mean cost at 30 days of $20,435 to $13,306 per patient) – in addition to improved patient satisfaction.2
- GDT: In a study of 82 patients undergoing major abdominal surgery, researchers found that GDT with fluid management using PVi reduced the volume of intraoperative fluid infused and reduced intraoperative and postoperative lactate levels.3
In addition, PVi has been used in combination with Masimo's noninvasive, continuous hemoglobin monitoring technology (SpHb®). In a study published last year involving 18,716 surgical patients at a hospital in Limoges, France, researchers investigated the effects of implementing a hospital-wide protocol for fluid management and blood administration using PVi and SpHb. The researchers found that use of an integrated GDT algorithm using both PVi and SpHb led to earlier transfusion and fewer units of blood transfused, as well as a 33% lower mortality rate 30 days after surgery and a 29% lower mortality rate 90 days after surgery. A year after the study ended, when the hospital did not use PVi and SpHb technology, the mortality rate returned to levels similar to those found before implementation of the GDT protocol.4
Stephen Weston, MD, Health Sciences Associate Clinical Professor and Medical Director, Operating Room Support Services, Department of Anesthesia and Perioperative Care at the University of California, San Francisco, said, "At UCSF we display PVi on all of our Masimo pulse oximeters. PVi is a great tool for helping to assess the patient’s fluid status. Having a fluid responsiveness monitor incorporated into something as ubiquitous as pulse oximetry allows us to monitor fluid responsiveness universally. I integrate PVi into my routine sweeps of the hemodynamic data in the operating room, and we’ve rolled out this functionality to the intensive care units as well. Without PVi, I feel that I’m flying a little bit blind."
Joe Kiani, Founder and CEO of Masimo, said, "We are thrilled that at long last we are able to offer clinicians and patients in the U.S. the full benefits of PVi monitoring. Multiple studies have shown how this breakthrough noninvasive indicator of fluid responsiveness can help improve outcomes and reduce costs on mechanically ventilated patients – with no more equipment needed than a software upgrade with most existing Masimo SET® and rainbow® platforms and the existing Masimo SET® sensors that so many top hospitals around the world already use for pulse oximetry."
The accuracy of PVi in predicting fluid responsiveness is variable and influenced by numerous patient, procedure, and device-related factors. PVi measures the variation in the plethysmography amplitude but does not provide measurements of stroke volume or cardiac output. Fluid management decisions should be based on a complete assessment of the patient’s condition and should not be based solely on PVi.
SpHb is not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician’s judgment considering, among other factors, patient condition, continuous SpHb monitoring, and laboratory diagnostic tests using blood samples.
The GDT and ERAS studies summarized above were conducted on patients undergoing specific types of procedures and following specific fluid management protocols. The results may not be reflective of all cases and the described GDT and ERAS protocols may not be appropriate for all types of patients and procedures.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.7 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,8 improve CCHD screening in newborns,9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.10-13 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,14 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.15 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Bloom J, Wyler D, Torjman M, Trinh T, Li L, Mehta A, Fitchett E, Kastenberg D, Mahla M, Romo V. High Incidence of Burst Suppression during Propofol Sedation for Outpatient Colonoscopy: Lessons Learned from Neuromonitoring. Anesthesiology Research and Practice. 2020;7246570. https://doi.org/10.1155/2020/7246570.
- Hughes, C, McGrane S, Pandharipande P. Sedation in the intensive care setting. Clinical pharmacology: advances and applications 4 (2012): 53.
- Fritz, B et al. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesth Analg. 122.1 (2016): 234.
- Hesse S et al. Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. BJA 122.5 (2019): 622-634.
- Purdon P et al. Clinical electroencephalography for anesthesiologists part I: background and basic signatures. Anesthesiology. 123.4 (2015): 937-960.
- Sayed E, Refaat E, Yassen K. SedLine Monitored Sedation and Recovery for Postoperative Ventilated Recipients of Living Donor Liver Transplantation: A Randomized Controlled Trial. J Anesth Clin Res 6.530 (2015): 2.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

New Blinded Observational Study Shows Greater Depth of Patient Sedation Than Intended During Procedural Colonoscopy
Masimo SedLine® Brain Function Monitoring with PSi Processed EEG Helped Identify the Oversedations
Irvine, California – August 3, 2020 - Masimo (NASDAQ: MASI) today announced that in a new study published in Anesthesia Research and Practice, researchers used Masimo SedLine® Brain Function Monitoring to investigate the depth of anesthesia of patients receiving propofol for outpatient colonoscopy, concluding that, "Although providers planned for moderate to deep sedation, processed EEG [electroencephalography] showed patients were under general anesthesia, often with burst suppression. Anesthesiologists and endoscopists may utilize processed EEG to recognize their institutional practice patterns of procedural sedation with propofol and improve upon it."1
|
Masimo SedLine® Brain Function Monitoring |
Noting that depth of sedation is "rarely quantified," but that "the spectrum of sedation actually attained in gastrointestinal endoscopy procedures may extend to levels deeper than planned for, thereby exposing patients to…cardiorespiratory risks," Dr. Jamie Bloom and colleagues at Thomas Jefferson University Hospital in Philadelphia sought to understand how frequently patients whose procedures called for moderate to deep sedation using propofol actually experience unintended general anesthesia and burst suppression. To quantify this, they monitored 119 adult patients undergoing outpatient colonoscopies using Masimo SedLine brain function monitoring, including its processed EEG index, the Patient State Index (PSi). PSi provides values that range from 0 to 100, with higher values indicating lesser degrees of sedation (with values from 50 to 25 indicative of general anesthesia, and below 25 for burst suppression). For the study, PSi values correlating to general anesthesia and burst suppression were confirmed by examination of the raw EEG by a neurointensivist and a neurophysiologist.
The researchers found that 118 of the 119 (99.1%) patients attained PSi values of <50, consistent with general anesthesia, and that these patients spent a significantly greater percentage of the procedure with PSi values <50 (53.1% of the time) vs. >50 (42% of the time) (p = 0.001). Of the 118 patients, 33 (27.7%) attained PSi values of <25, consistent with burst suppression. In addition, the researchers found the mean total dose of propofol was significantly correlated with PSi during periods of PSi values <25 (R = 0.406, p = 0.021).
Based on the study results, the researchers concluded, "The depth of sedation achieved with anesthesia administered propofol for colonoscopy spans a continuum. Although providers planned for moderate to deep sedation, processed EEG in this study revealed a substantially greater depth consistent with general anesthesia and even burst suppression."
They continued, "Feedback from devices such as employed in this study to guide the depth of sedation may be considered to not only raise awareness of the physiologic implications of deeper levels of sedation but also to optimize institutional sedation practice. Further research is required to establish the impact on patient outcomes of anesthetic practices resulting in intraprocedural burst suppression."
Patients receiving anesthesia during surgery can be over- or undersedated, both of which carry risks. When under-anesthetized, patients may have awareness during the procedure, which can be traumatic; when they are over-anesthetized, they may have alternating periods of no brain activity as measured by EEG, which is referred to as burst suppression.2 Burst suppression has been correlated with increased risk of postoperative delirium,3 which in turn has been shown to increase hospital length of stay and readmission rates, thus increasing costs.4 Burst suppression has also been associated with postoperative trauma, dementia, decreased quality of life, additional healthcare costs, and even death.3 In addition to burst suppression, overly deep sedation may result in loss of spontaneous ventilation and impaired cardiovascular function.1 Oversedation has also been associated with longer time on mechanical ventilation, longer time in the ICU, greater need for radiological evaluation of mental status, and a higher probability of developing brain dysfunction.2
The role of anesthesiologists in keeping patients appropriately sedated, and not over- or under-sedated, is critically important. Masimo SedLine has been shown to help anesthesiologists understand the patient’s sedation state using not only PSi values, but also through interpretation of the raw EEG and the Density Spectral Array that SedLine provides.4-6 In the current study, researchers showed that even in outpatient procedures, like colonoscopy, there is still a risk of patients being over-anesthetized, leading to burst suppression, which was identified using the PSi available as part of Masimo SedLine brain function monitoring.1
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.5 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,6 improve CCHD screening in newborns,7 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.8-11 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,12 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.13 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on PVi, with varying results and outcomes, can be found on our website at https://www.masimo.com/evidence/pulse-oximetry/pvi. Studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Thiele RH et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015 Apr;220(4):430-43.
- Forget P et al. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010 Oct;111(4):910-4.
- Cros J et al. Continuous hemoglobin and plethysmography variability index monitoring can modify blood transfusion practice and is associated with lower mortality. J Clin Monit Comp. 3 Aug 2019. https://doi.org/10.1007/s10877-019-00367-z.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo PVi® and SpHb®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo PVi and SpHb, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Named NBA Supplier of Pulse Oximetry
NBA Deploys MightySat® Fingertip Pulse Oximeters For All Campus Residents
Irvine, California – July 30, 2020 - Masimo (NASDAQ: MASI) today announced that it has entered into a partnership with the National Basketball Association (NBA) to be its official supplier of pulse oximetry. As teams return to play, Masimo is supplying the NBA with MightySat® fingertip pulse oximeters on its campus in Orlando, Florida.
|
Gary Harris of the Denver Nuggets with Masimo MightySat® |
Since arriving on campus in Orlando on July 7, and for the duration of their stay, each campus resident, including players, coaches, and team and league staff, will daily measure their oxygen saturation, pulse rate, and respiration rate with MightySat as part of the league’s health and safety protocols. MightySat is powered by clinically proven, hospital-grade Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry technology.
Dr. John DiFiori, NBA Director of Sports Medicine, said, “Fingertip pulse oximetry is an important component of the health and safety protocols that govern the NBA’s re-start in Orlando. Our players, teams and staff have been quick to adapt to the Masimo technology to monitor their oxygen saturation rates, which is contributing to keeping everyone on our campus safe and healthy.”
Gary Harris, shooting guard for the Denver Nuggets, added, “I’ve personally been using the MightySat in my training these past few years. It’s incredibly accurate and has helped me get the most out of my workouts and recovery. I’m glad to see it as part of our health monitoring program as our team gets ready to resume the 2020 season.”
Joe Kiani, Founder and CEO of Masimo, said, “We are thrilled to have the opportunity to provide our high performance monitoring technology to such high performance athletes. For more than 30 years, we’ve specialized in innovative breakthrough hospital monitoring solutions, and we are excited to continue our expansion into new markets, bringing hospital-grade health and wellness monitoring solutions to everyday users, elite athletes, and organizations like the NBA.”
The NBA will re-start its season today at ESPN Wide World of Sports in Orlando, Florida. The 22 teams with the best records as of March 11, when the season was suspended, will compete in eight preliminary “seeding” games before the 2020 NBA Playoffs begin on August 17.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2020-21 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo SafetyNet™, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo MightySat®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo MightySat, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]

Masimo Expands the Masimo SafetyNet™ Remote Patient Management Solution with Continuous Body Temperature Measurement
The Radius T°™ Wearable Temperature Sensor Automates Remote Monitoring of Patient Temperature Status
Irvine, California – July 27, 2020 - Masimo (NASDAQ: MASI) today announced a significant expansion to the Masimo SafetyNet™ platform with the introduction of Radius T°™, a wearable, wireless sensor that provides continuous body temperature measurements. By augmenting the already powerful Masimo SafetyNet, which features Radius PPG™ tetherless pulse oximetry, with Radius T°, the remote patient management solution becomes capable of tracking four key vital signs – oxygen saturation, respiration rate, pulse rate, and now temperature – making it an ideal solution for assessing the status of patients with suspected or low-acuity COVID-19, among many other remote patient management uses. Unlike spot-check thermometry solutions, Radius T° measures body temperature continuously, providing remote notifications when a patient’s temperature is outside a clinician-specified range – giving peace of mind to caregiver and patient alike.
|
|
Masimo SafetyNet uses a tetherless, wearable single-patient-use Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry sensor to monitor a patient's blood oxygen saturation and pulse rate, as well as respiration rate, perfusion index, and PVi®. Masimo SafetyNet is designed to help manage the surge in COVID-19 patients while maintaining distance from other patients and providers, allowing hospitals to expand remote patient monitoring into alternative care spaces, including overflow locations, emergency recovery facilities, and home care settings. The telehealth platform combines tetherless pulse oximetry—and now also Radius T° continuous temperature measurement – with a cloud-based remote data capture and surveillance platform accessible from a patient's Android or iOS smartphone or smart device. Monitoring key physiological data provides clinicians with the ability to assess patient status and facilitates awareness of the need for intervention.
Temperature measurement by patients at home typically occurs intermittently, at the prompting of a clinician, and is prone to poor compliance in the collection and reporting of data to healthcare providers in a timely, consistent fashion. A patient taking their temperature at regular or semi-regular intervals may only notice a spike in temperature hours after a fever has begun, or may not even be aware of it, delaying possible clinical intervention. In contrast, Radius T° collects data continuously and seamlessly, recording trend data and automatically notifying remotely when a clinician-specified high temperature threshold is breached, without any action needed on the patient's part. By eliminating inconsistent manual measurements and concerns about patient compliance, while providing continuous insight into changes in body temperature, Radius T° is intended to significantly improve patient status assessment workflows.
Radius Tº is small, light, and comfortable, and is easily applied to the chest. Each shower-proof, single-patient-use sensor lasts up to 8 days and can be worn throughout the day and night, allowing patients to continue normal daily activities while still being monitored. Applied to the skin, Radius T° uses proprietary algorithms to measure the patient’s body temperature, not just external skin temperature, with laboratory accuracy within ±0.1ºC, whereas other thermometry solutions typically have laboratory accuracy within ±0.2ºC. Using Bluetooth®, Radius T° provides this continuous data to the Masimo SafetyNet app on the patient's smartphone and via secure cloud to clinicians back at the hospital, allowing them to track and trend a patient's body temperature, helping them to spot potential deterioration in patient status using the web-based Masimo SafetyNet Clinician Portal.
Joe Kiani, Founder and CEO of Masimo, said, "We're proud to add this noninvasive, continuous, wearable thermometer solution to our growing family of remote patient management solutions. Masimo SafetyNet has already helped clinicians effectively care for countless patients during the pandemic. With the addition of Radius T°, Masimo SafetyNet becomes an even more useful tool for remotely managing patients with COVID-19 and many other health concerns."
Radius T° is indicated for use on patients 5 years and older. Radius T° is not FDA 510(k) cleared; the device is marketed under the FDA's Enforcement Policy for Clinical Electronic Thermometers During COVID-19.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™ and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer A et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath S et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- McGrath S et al. Inpatient Respiratory Arrest Associated With Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 2020 14 Mar. DOI: 10.1097/PTS.0000000000000696.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™ and Radius T°™. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet and Radius T°, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

New Study Expands Evidence Demonstrating the Benefits of Critical Congenital Heart Disease (CCHD) Screening Using Masimo SET® Pulse Oximetry
Neuchatel, Switzerland – July 20, 2020 - Masimo (NASDAQ: MASI) today announced the results of a prospective study published in the International Journal of Neonatal Screening in which researchers in Marrakesh, Morocco conducted the first Moroccan study on critical congenital heart disease (CCHD) screening for newborns using Masimo SET® pulse oximetry.1 The authors concluded that "Our results encourage us to strengthen screening for CCHD by adding pulse oximetry to the routine newborn screening panel."
|
|
Dr. Slitine and colleagues sought to improve early detection of CCHD in Morocco by studying the feasibility of implementing CCHD screening using pulse oximetry. From March 2019 to January 2020, 8013 asymptomatic newborns at Mother and Child Hospital (part of the Mohammed VI University Hospital of Marrakesh), who were "normal" according to neonatal examination using the current standard, were screened for CCHD in accordance with American Academy of Pediatrics (AAP) guidelines, including pre- and post-ductal oxygen saturation measurement, using Masimo Rad-97® and Radical-7® Pulse CO-Oximeters® with Masimo SET® pulse oximetry sensors.
The researchers found that, of the 8013 infants screened, 7998 newborns had a negative screen (99.82%) and 15 newborns were screen positive (0.18%). Of those 15, five were later diagnosed with CCHD and five with non-critical CHD; five were false positives (three of which had other underlying conditions). Of the 7998 infants who passed, there was one false negative, an infant who was later diagnosed, at 2 months of age, with coarctation of the aorta.
The researchers noted that the screening test was "easy, simple, reliable, reproducible, acceptable, discriminating, well-accepted by parents and caregivers, and did not involve parental anxiety." They also noted that "Pulse oximetry has a good specificity and sensitivity and thereby fulfills the criteria for screening. Additionally, most of the data in the literature suggest a favorable cost-effectiveness of this technique."
The authors concluded, "Screening for CCHD is a reliable method for the early detection of critical congenital heart disease and even non-cardiac conditions. We think that it will have positive repercussions on infant mortality and morbidity in Morocco. It is an optimal test and it adapts perfectly to our context. We hope to implement it locally and nationally, as consistent with international best practice for newborn screening, to allow for timely detection of the infants born with CCHD in Morocco."
Although the researchers at times use the term pulse oximetry generally, the specific pulse oximetry technology used in this study, as noted, was Masimo SET®. To date, six other large published CCHD screening studies,2-7 as well as additional, smaller studies,8-9 have used Masimo SET®. Cumulatively, the large studies represent 284,800 infants, which includes the largest CCHD study to date, of 122,738 newborns.2 All of these CCHD studies with Masimo SET® pulse oximetry showed improved screening sensitivity with the use of Masimo SET® alongside clinical assessment when compared to routine physical exam alone. With its ability to accurately measure through motion and low perfusion, alongside its performance in outcome studies, Masimo SET® stands out as the established choice of pulse oximetry technology for clinicians and policy makers hoping to implement CCHD screening processes.10
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.10 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,11 improve CCHD screening in newborns,2-9 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.12-14 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,15 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.16 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™ and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Slitine N, Bennaoui F, Sable C, Martin G, Hom L, Fadel A, Moussaoui S, Inajjarne N, Boumzebra D, Mouaffak Y, Younous S, Boukhanni L, Maoulainine F. Pulse Oximetry and Congenital Heart Disease Screening: Results of the First Pilot Study in Morocco. Int J Neonatal Screen 6(53). 30 June 2020.
- Zhao et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014 Aug 30;384(9945):747-54.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Ewer AK et al. Pulse Oximetry Screening for Congenital Heart Defects in Newborn Infants (Pulseox): A Test Accuracy Study. Lancet. 2011 Aug 27;378(9793):785-94.
- de-Wahl Granelli A et al. Noninvasive Peripheral Perfusion Index as a Possible Tool for Screening for Critical Left Heart Obstruction. Acta Paediatr 2007; 96(10): 1455-9.
- Meberg A et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. J Pediatr 2008; 152:761-5.
- Schena F et al. Perfusion Index and Pulse Oximetry Screening for Congenital Heart Defects. J Pediatr. 2017 Apr;183:74-79.
- Hamilçıkan S, Can E. Critical Congenital Heart Disease Screening With a Pulse Oximetry in Neonates. J Perinat Med. 2018 Feb 23;46(2):203-207.
- Jawin V et al. Beyond Critical Congenital Heart Disease: Newborn Screening Using Pulse Oximetry for Neonatal Sepsis and Respiratory Diseases in a Middle-Income Country. PLoS One. 2015; 10(9): e0137580.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Centroid™ and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Centroid and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces UniView: 60™ Digital Charting Solution
UniView: 60 Uses the Power of the Masimo Hospital Automation™ Platform to Improve Clinical Workflows and Hand-off Communication
Irvine, California – July 14, 2020 - Masimo (NASDAQ: MASI) today announced its latest automation and connectivity solution, UniView: 60™. UniView: 60 uses the Masimo Hospital Automation™ platform to aggregate and display pertinent patient information on a digital display just outside each patient's room, allowing clinicians to familiarize themselves with the most relevant details of each patient’s case at the door in 60 seconds or less before they see the patient.
|
|
UniView: 60 is intended to improve clinical workflows by helping increase clinicians' awareness of important patient information and advisories – for example, a patient’s risk of falling, environmental warnings, lab results, prescription history, radiological images, monitoring data, and much more – optimally displayed, based on hospital preferences, to help clinicians quickly interpret pertinent information. Unlike paper records and notes, which may display outdated or incorrect information, UniView: 60 utilizes its connections to various hospital information systems to display the most appropriate, up-to-date information directly outside the patient’s room in order to increase clinical efficiency and improve handoff communication between staff.
The hand-off communication process represents an opportunity for significant improvements in patient safety. When clinical information is absent, incomplete, erroneous, or delayed, it can lead to serious patient harm, including medication errors, inaccurate patient plans, delays in transfer to critical care, delays in discharge, and repetitive testing.1 Adopting a clear and consistent communication strategy, as leading institutions like the University of California, Irvine (UCI) Medical Center have done, has been shown to reduce ineffective hand-offs by nearly 60%.2 UniView: 60 is designed to be part of just such a strategy to improve patient safety: by incorporating patient monitoring data and other data and advisories in a standardized fashion from each patient’s full EMR history, UniView: 60 can help to make the hand-off process more consistent and effective.
UniView: 60 is one of many innovations that Masimo is developing to take advantage of the powerful connectivity and data handling architecture of the Masimo Hospital Automation platform, which connects systems across the continuum of care, from bedside monitors and connected third-party devices to electronic medical records (EMRs). Powered by ACE™, advanced translation capabilities, and a robust automation engine, the Masimo Hospital Automation platform helps to ensure interoperability and the free, secure movement of data throughout hospital systems. UniView: 60 takes advantage of this flow of data by optimizing the presentation of pertinent information about each patient's case on an easy-to-interpret, customizable display.
As part of its vision of the hospital of the future and to further its longstanding commitment to patient safety, UCI Medical Center has chosen to deploy UniView: 60 in its new acute care unit. The new unit features the Masimo Root® Patient Monitoring and Connectivity Platform with Radius-7® wearable Pulse CO-Oximeters® at every bedside, Patient SafetyNet™ for supplemental remote monitoring and clinician notification – and at the entrance to each room, UniView: 60 digital chart displays.
"When UCI Health planned its new acute care unit at UCI Medical Center, we wanted a system that allows the entire care team to quickly see and understand a patient's most critical information in a way that enhances patient safety and their relationship with the patient as an individual," said UCI Health CEO Chad T. Lefteris. "Today's clinical environment generates so much data that physicians, nurses, therapists, and other staff can lose sight of the patient. Masimo’s Hospital Automation platform and UniView: 60 meet our objectives by providing the comprehensive solution UCI Health needed to give our patients the best experience possible, while reinforcing our commitment to improving patient outcomes and safety."
Joe Kiani, Founder and CEO of Masimo, said, "We’re delighted to announce UniView: 60 and be able to deploy it at one of our excellent local institutions, the University of California Irvine Medical Center. We're proud to have been able to help UCI as they seek to build the hospital of the future. With UniView: 60, their clinicians are experiencing the power of the Masimo Hospital Automation platform, which connects data and automates processes throughout the hospital, and which is now being harnessed to help ensure that patients get the right care."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.3 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,4 improve CCHD screening in newborns,5 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.6-8 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,9 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.10 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Masimo Hospital Automation™ platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, UniView: 60™ and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Flemming D and Hübner U. How to Improve Change of Shift Handovers and Collaborative Grounding and What Role Does the Electronic Patient Record System Play? Results of a Systematic Literature Review. Int J Med Inform. 2013 Jul;82(7):580-92..
- Benjamin M, Hargrave S, and Nether K. Using the Target Solution Tool to Improve Emergency Department Handoffs in a Community Hospital. Jt Comm J Qual Patient Saf. 2016 Mar;42(3):107-18.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
About UCI Medical Center
UCI Health comprises the clinical enterprise of the University of California, Irvine. Patients can access UCI Health at primary and specialty care offices across Orange County and at its main campus, UCI Medical Center in Orange, California. The 418-bed acute care hospital provides tertiary and quaternary care, ambulatory and specialty medical clinics, and behavioral health and rehabilitation services. UCI Medical Center features Orange County’s only National Cancer Institute-designated comprehensive cancer center, high-risk perinatal/neonatal program and American College of Surgeons-verified Level I adult and Level II pediatric trauma center and regional burn center. It is the primary teaching hospital for the UCI School of Medicine. UCI Health serves a region of nearly 4 million people in Orange County, western Riverside County and southeast Los Angeles County. Follow us on Facebook and Twitter.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Centroid™ and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Centroid and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact - Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Media Contact - UCI Health
John Murray
Phone: (714) 456-7759
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Study Investigates Accuracy of Respiration Rate Obtained from Pulse Oximetry on Pediatric Patients Using Masimo RRp®
Neuchatel, Switzerland – July 06, 2020 - Masimo (NASDAQ: MASI) today announced the results of a study published in Pneumonia in which independent researchers in New Delhi, India, investigated the accuracy of Masimo RRp® on pediatric patients with the Rad-G™ Pulse Oximeter by comparing it to clinician-determined values while performing routine assessment of children admitted to outpatient and emergency departments.1 RRp provides respiration rate determined from the photoplethysmograph used in pulse oximetry
|
|
Noting the high incidence of childhood pneumonia in many parts of the world, the inclusion of oxygen saturation (SpO2) and respiratory rate measurement in pneumonia screening guidelines, and the scarcity of medical equipment and variability in medical training in many low-resource settings, Dr. Alwadhi and colleagues sought to determine whether a "multi-modal" pulse oximeter, Masimo Rad-G, could also accurately measure respiration rate – supporting future applications for a more streamlined and reliable approach to pneumonia screening case management. Rad-G uses a single Masimo SET® pulse oximetry sensor to measure both SpO2 and RRp, as well as pulse rate (PR), perfusion index (Pi), and pleth variability index (PVi®).
In this particular study, the researchers used Rad-G, alongside traditional pediatrician assessment, to measure the respiration rate of 97 children (aged 2 to 59 months) admitted to the outpatient and emergency departments at Kalawati Saran Hospital in New Delhi, over 2 weeks. They then analyzed the level of agreement between plethysmography-based respiration rate (RRp) and the clinicians' assessment of respiration rate.
The researchers found that RRp and the control respiration rate measurement method showed "significant strong association (97%)" (p < 0.001), and that "values obtained either from pulse oximeter or by pediatrician (gold standard) are very close to each other." For assessing fast breathing (defined as ≥ 50 breaths per minute (bpm) for infants 2 to 12 months and ≥ 40 bpm for children older than 12 months), RRp had high sensitivity (95%) and specificity (94%), with 95% accuracy. Based on these findings, the authors explained, "The sensitivity analysis, in addition, points to the reliability of the device in correctly identifying fast breathing, a major symptom of the disease [pneumonia], in 95% of the cases." They also noted that Rad-G "allows for significantly integrated results of RR and SpO2 with high sensitivity and accuracy in health care settings, as well as the possibility of rapid detection."
Joe Kiani, Founder and CEO of Masimo, said, "The opioid epidemic continues to impact millions of people around the world. Unassisted withdrawal from long-term use can be lengthy, extremely unpleasant, and there is a high risk of relapse. Effectively helping to reduce opioid withdrawal symptoms, which is what Bridge has been shown to do, is a critical first step toward successful illicit opioid cessation and treatment. This is the first tool that we are introducing to help with the opioid epidemic. We are committed to doing what we can to help reduce opioid-related deaths."
The researchers concluded, "There is a high degree of agreement between pleth-based RR using a [pulse oximetry device] and physician measured RR, indicating that the former provides reliable and accurate measurement. Current diagnosis and management of pneumonia in primary health care is based on variably trained health providers despite IMCI [Integrated Management for Childhood Illness] guidelines. The use of pulse oximeters, also recommended by the WHO, which can provide reliable measurement, would streamline pneumonia case management in these settings. The current study provides evidence of the reliability of pulse oximeter[s]."
Masimo RRp is CE marked and U.S. FDA 510(k) cleared. Rad-G is currently CE marked and the U.S. FDA 510(k) clearance is pending.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO9 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Alwadhi V, Sarin E, Kumar P, Saboth P, Khera A, Gupta S, Kumar H. Measuring accuracy of plethysmography based respiratory rate measurement using pulse oximeter at a tertiary hospital in India. Pneumonia. 2020. 12:4. https://doi.org/10.1186/s41479-020-00067-2.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Centroid™ and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Centroid and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Bridge™
Drug-free Neurostimulation Device Reduces the Symptoms of Opioid Withdrawal
Irvine, California – June 29, 2020 - Masimo (NASDAQ: MASI) today announced Bridge™, an opioid withdrawal solution that uses neuromodulation to aid in the reduction of symptoms associated with opioid withdrawal. Bridge, which has been granted an FDA De Novo classification, is the first evidence-based, drug-free, non-surgical device of its kind.
|
|
Opioid withdrawal is often accompanied by painful, often severe flu-like symptoms lasting for up to two weeks, making voluntary discontinuation by opioid users a long, challenging process. For many patients with opioid-use disorder (OUD), fear of the physical and psychological effects of withdrawal can be a barrier to even attempting to seek treatment. It has been estimated that less than 20% of the more than two million people suffering from OUD in the United States are receiving treatment.1
By aiding in the reduction of withdrawal symptoms, Bridge can help patients with OUD successfully transition away from opioids into an appropriate treatment program, helping them safely address their disorder. In clinical testing, Bridge was found to reduce opioid withdrawal symptoms within 15-30 minutes and provide continuous relief for as long as it was applied, which can be up to 120 hours per device, allowing opioids to leave the body. In a study of 73 adult OUD patients, it was shown that withdrawal symptoms (such as increases in resting pulse rate, sweating, restlessness, bone or joint aches, tremors, and anxiety) were reduced by 85% after the first hour of using the device and 97% after 5 days of use (measured using the clinical opiate withdrawal scale).2
The solution consists of a wearable, single-patient-use, percutaneous neurostimulator, fitted behind the ear, which applies gentle electrical impulses to branches of the cranial nerves around the ear. These electrical impulses to the cranial nerves have been found to reduce heightened neuron activity,3 which is associated with opioid withdrawal symptoms. Bridge can be easily applied by a healthcare professional in a simple, non-surgical, in-office procedure, and its use doesn’t interfere with normal daily activities such as work or school.
Joe Kiani, Founder and CEO of Masimo, said, "The opioid epidemic continues to impact millions of people around the world. Unassisted withdrawal from long-term use can be lengthy, extremely unpleasant, and there is a high risk of relapse. Effectively helping to reduce opioid withdrawal symptoms, which is what Bridge has been shown to do, is a critical first step toward successful illicit opioid cessation and treatment. This is the first tool that we are introducing to help with the opioid epidemic. We are committed to doing what we can to help reduce opioid-related deaths."
Opioid use (including prescription opioids and heroin) kills many people in the U.S. each year. From 1999 to 2018, opioids were involved in more than 446,000 deaths,4 approximately 232,000 of which were the result of overdoses related to prescription opioids.5 In 2018, the most recent year for which data is available, approximately 47,000 deaths involved opioids, almost 15,000 of which involved prescription opioids.4
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO9 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- https://www.drugabuse.gov/publications/research-reports/medications-to-treat-opioid-addiction/overview
- Miranda A, Taca A. Neuromodulation with percutaneous electrical nerve field stimulation is associated with reduction in signs and symptoms of opioid withdrawal: a multisite, retrospective assessment. Am J Drug Alcohol Abuse. 2017 Mar 16;1-8.
- Babygirija R et al. Percutaneous Electrical Nerve Field Stimulation Modulates Central Pain Pathways and Attenuates Post-inflammatory Visceral and Somatic Hyperalgesia in Rats. Neuroscience 356 (2017) 11-21.
- Wilson N et al. Drug and Opioid-Involved Overdose Deaths — United States, 2017–2018. MMWR Morb Mortal Wkly Rep 2020;69:290–297. DOI: https://dx.doi.org/10.15585/mmwr.mm6911a4.
- https://www.cdc.gov/drugoverdose/data/prescribing/overview.html.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Centroid™ and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Centroid and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces FDA Clearance of Centroid™
Wearable, Wireless Patient Orientation, Activity, and Respiration Sensor Helps Clinicians Monitor Patient Position and Respiration Rate
Irvine, California – June 25, 2020 - Masimo (NASDAQ: MASI) announced today that Centroid™, a wearable, wireless patient orientation, activity, and respiration rate sensor, has received FDA clearance. Centroid helps clinicians monitor patient position to avoid preventable pressure ulcers, and can alert clinicians to sudden movements such as fall-like events. In addition, Centroid detects chest movements to continuously provide respiration rate, assisting clinicians with additional data that may inform care decisions.
|
|
Centroid pairs with the Root® Patient Monitoring and Connectivity Platform using Bluetooth® to track a patient’s posture, orientation, and activity, providing the ability to monitor patient position and detect changes in position. The data transmitted by Centroid can be displayed in various formats on Root, giving clinicians multiple ways to assess adherence to protocols regarding tissue stress and to tailor care to the specific needs of each patient.
Pressure sores affect nearly 2.5 million patients per year in U.S. hospitals alone, and approximately 60,000 of those patients die as a direct result.1 Centroid is indicated for the orientation monitoring of patients who may be susceptible to pressure ulcers, by tracking patient movement and activity using an accelerometer and gyroscope. Centroid can identify a patient’s position and orientation to the nearest degree, with alerts based on the duration in a static position to help clinicians adhere to hospital patient turn protocols. Centroid also features customizable alarm zones to help avoid patient positions that could negatively impact recovery time. Unlike simple time-based rotation protocols, Centroid uses the cumulative time spent in each position, as well as existing sore data, to calculate relative risk, displayed on the Root screen using color-coded markers, helping clinicians identify the potential severity of tissue stress for each position—and ultimately, helping to guide clinical decisions about the most appropriate, least risky positions for each patient.
In addition, because Centroid can identify whether a patient is lying down, standing, sitting upright, walking, or may have fallen, it can notify clinicians of a sudden change in position that might provide early warning of a potential fall, by alerting them when clinician-defined movement thresholds are crossed. Its respiration rate performance, validated against manually scored capnogram respiratory measurements, is accurate to within 3 respirations per minute (rpm) in the range of 8 to 35 rpm.
The Centroid single-patient-use sensor is ergonomically designed for application on the chest using flexible, lightweight material for patient comfort, with a gentle adhesive that supports continuous use during daily activities. Each battery-operated sensor is designed to last four days, minimizing the need for frequent replacement.
Joe Kiani, Founder and CEO of Masimo, said, “We are committed to using our expertise in signal processing and sensor design to develop new ways to provide the highest quality, most relevant data to clinicians in the most intuitive, useful formats, and Centroid, coupled with Root’s rich high-resolution display, is a great example of this. We hope that by helping to automate the process of tracking and making decisions about patient position, we can help clinicians reduce the frequency and severity of pressure ulcers, and ultimately improve patient outcomes.”
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO9 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Preventing Pressure Ulcers in Hospitals. Agency for Healthcare Research and Quality. https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html. Accessed 23 Apr 2020.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Centroid™ and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Centroid and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Masimo SafetyNet-Open™
Suite of Integrated Turnkey Tools Helps Businesses, Governments, and Schools Reopen and Stay Open Responsibly During COVID-19
Irvine, California - June 19, 2020 – Masimo (NASDAQ: MASI) today announced Masimo SafetyNet-Open™, designed to help businesses, governments, and schools more responsibly manage employee and student health and safety during COVID-19. As the pandemic continues, companies and organizations worldwide struggle to find the appropriate balance between reopening and keeping people safe by reducing the risk of infection. As a global leader in noninvasive patient monitoring technologies and advanced connectivity and automation solutions, Masimo is uniquely positioned to provide organizations with tools to assist them in reopening safely.
|
|
Trusted by leading hospitals to monitor more than 200 million patients each year,1 Masimo, in the early phases of the pandemic, developed the Masimo SafetyNet-COVID-19™ remote patient monitoring solution, which is already being used by hospitals to help keep not just their patients, but also their frontline workers safe. Masimo SafetyNet-Open helps address the challenge of reopening responsibly and safely with a comprehensive, flexible, and easy-to-deploy continuous monitoring solution that, in conjunction with clinical guidance from partner hospitals, helps manage prevention, early identification, and recovery monitoring.
Much more than a simple screening check at the workplace entrance, Masimo SafetyNet-Open helps coordinate the continuous monitoring of people through a variety of sensors and an app. Using a centralized dashboard, Masimo SafetyNet-Open can help organizations better understand patterns that may support contact tracing. The system components work together to provide integrated and secure monitoring that organizations can use to identify when additional actions may be needed.
Joe Kiani, Founder and CEO of Masimo, said, "As the COVID-19 pandemic continues, businesses, governments, and schools around the world have to make difficult risk analysis decisions that balance the health and safety of their constituents with their communities' needs. We are not in the position to suggest when it’s safe to reopen, but we are in the unique position to help those who are going to open, to open as safely as possible – and hopefully help them stay open."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Estimate: Masimo data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Sleep™, a New Wellness Solution That Allows Users to Better Understand Their Sleep Quality
Irvine, California - June 19, 2020 – Masimo (NASDAQ: MASI) announced today a new health and wellness home monitoring solution, Masimo Sleep™, designed to help consumers better understand the quality of their sleep. Masimo Sleep is fueled by the same expertise in signal processing and sensor development that drives the Masimo hospital products used by leading institutions to monitor over 200 million patients a year.1 Masimo Sleep is available for pre-order starting today at www.masimo.com.
|
|
Masimo Sleep, a wearable sleep quality solution based on clinically proven vital signs technology, is unlike any other sleep management solution available to consumers. Masimo Sleep analyzes your data to provide truly personalized multi-dimensional insight into your sleep health – empowering you with actionable guidance to help you make healthier lifestyle choices that improve your sleep.
The user wears a lightweight sensor (for up to ten nights per sensor), while the Sleep app collects the user’s data using clinically proven Masimo SET® pulse oximetry to track changes in key vital signs during sleep. By analyzing oxygenation, heart rate, and respiratory rate overnight, Masimo Sleep can provide guidance to help users better understand, for example, why they may snore or wake up tired, and recommend ways for the user to improve the quality of their sleep. Other at home products look at proxies for healthy sleep, like how often users roll over, or listen to the sound of users' breathing and snoring, rather than their vital signs. Masimo Sleep helps people to better understand their physiological status overnight – from the comfort of their own bed.
Joe Kiani, Founder and CEO of Masimo, said, "We are delighted to unveil Masimo Sleep as part of our line of direct-to-consumer solutions, which also includes the MightySat® fingertip pulse oximeter. Based on our expertise in monitoring the physiological status of hundreds of millions of patients each year, Masimo Sleep significantly expands consumers' ability to make healthier decisions, at home, by equipping them with what we believe is the best solution for this need."
Masimo Sleep is not FDA 510(k) cleared. It is available as a general wellness product.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.2 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,3 improve CCHD screening in newborns,4 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.5-7 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,8 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.9 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Estimate: Masimo data on file.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo and Philips Licensing Agreement Brings Masimo NomoLine® Capnography and O3® Regional Oximetry to Select Philips Patient Monitors
Amsterdam, the Netherlands and Irvine California – Masimo (NASDAQ: MASI) and Royal Philips (NYSE: PHG; AEX: PHIA), both global leaders in patient monitoring, announced today that they have reinforced their partnership, whereby Philips will integrate additional Masimo measurement technologies into select IntelliVue® MX-series multi-parameter monitors, to help clinicians assess cerebral oximetry and ventilation status.
Core Masimo noninvasive measurements, such as SET® Measure-through Motion and Low Perfusion™ pulse oximetry and advanced rainbow SET® Pulse CO-Oximetry parameters like noninvasive hemoglobin (SpHb®), have long been available on a wide range of Philips multi-parameter monitors.
In 2016, Masimo and Philips entered into a multi-year business partnership involving both companies’ innovations in patient monitoring and therapy solutions. Now, in 2020, Philips becomes one of Masimo’s first industry partners to launch additional advanced Masimo measurements, NomoLine and O3, in their own patient monitors, sharing Masimo’s expertise in capnography and regional cerebral oximetry with caregivers and patients around the world.
NomoLine capnography and O3 regional oximetry measurements are available now on Philips MX500 and MX550 monitors [1] in select markets [2] throughout the world, and are also available directly from Masimo on its Root® Patient Monitoring and Connectivity Platform and when connected to select Philips patient monitors via Philips’ IntelliBridge module:
- NomoLine “no-moisture” sampling lines are designed for low-flow applications and can be used in a variety of clinical scenarios and care settings, on both intubated and non-intubated patients of all ages, in both low- and high-humidity applications.
- O3 regional oximetry may help clinicians monitor cerebral oxygenation in situations in which pulse oximetry alone may not be fully indicative of the oxygen in the brain.
“As global leader in health technology, we are delighted to reinforce our partnership with Masimo, bringing their advanced measurements to our innovative IntelliVue patient monitors, thereby making them even more versatile,” said Frans van Houten, CEO of Royal Philips. “Our customers expect integrated solutions to help them address the quadruple aim of healthcare. The complementary strengths of Philips and Masimo allow us to offer, reliable ventilation and regional oximetry solutions, which we expect will help clinicians deliver even better patient care.”
“Combining our expertise in noninvasive monitoring and signal processing technologies with Philips’ expertise in integrated patient monitoring and therapy solutions is a win-win for patients and clinicians alike,” said Joe Kiani, Founder and CEO of Masimo. “We are proud that Philips, recognizing our expertise in this clinical space, has chosen to make our innovative O3 and NomoLine platforms available to their customers. We look forward to continuing our partnership with a focus on improving patient outcomes and reducing the cost of care.”
- [1] Pending 510k, not available for sale in the USA
- [2] Not for sale in Albania, American Samoa, Bosnia and Herzegovina, Brazil, Belarus, Canada, China, Columbia, Costa Rica, Algeria, Egypt (O3 is available for sale), Guam, Hong Kong, Indonesia, , Iran, Japan, Kenya, Cambodia, Korea, Kazakhstan, Sri Lanka, Morocco (O3 is available for sale), Montenegro, Macedonia, Northern Mariana Islands, Mexico, Malaysia, Panama, Peru, Philippines, Puerto Rico, Serbia, Russian Federation, Saudi Arabia, Singapore, Syrian Arab Republic, Thailand, Turkey, Taiwan, Ukraine, Venezuela, U.S. Virgin Islands, Vietnam, South Africa.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips generated 2019 sales of EUR 19.5 billion and employs approximately 81,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies [1]. Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates [2], improve CCHD screening in newborns [3], and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs [4-6]. Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world [7], and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll [8]. Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient’s physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo’s family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview
Forward-Looking Statements: Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo NomoLine®, O3®, SET®, rainbow SET®, and Root®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo NomoLine, O3, SET®, rainbow SET®, and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. We do not undertake any obligation to update, amend or clarify these statements or the "Risk Factors" contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
For further information, please contact:
Steve Klink
Philips Global Press Office
Tel.: +31 6 10888824
Email: [email protected]
Evan Lamb
Masimo
Tel.: +1 949 396 3376
Email: [email protected]

Masimo and Samsung Partner to Package Masimo SafetyNet™ with Select Samsung Phones to Speed COVID-19 Response Efforts
Sensor-App-Phone Bundle Expected to Help Expedite Deployment of Remote Patient Management Solution to Patients Without Smartphones
Irvine, California - April 16, 2020 – Masimo (NASDAQ: MASI) announced today that it is partnering with Samsung Electronics America to make the Masimo SafetyNet™ Patient App available on select Samsung smartphones, pre-installed and pre-configured. Masimo SafetyNet is an innovative, economically scalable cloud-based patient management solution designed to help clinicians care for patients remotely in hospital settings and in non-traditional settings and circumstances. The partnership with Samsung is expected to allow for broader, faster distribution of Masimo SafetyNet to COVID-19 patients, especially older patients who are less likely to own a smartphone or have the comfort level to configure the Masimo SafetyNet App on their own.
Masimo's telehealth platform uses a tetherless, wearable single-patient-use SET® sensor to monitor a patient's blood oxygen saturation and pulse rate, as well as respiration rate, perfusion index, and PVi®, and is designed to help manage the surge in COVID-19 patients while maintaining distance from other patients and providers, allowing hospitals to expand patient remote monitoring into alternative care spaces, including overflow locations, emergency recovery facilities, skilled nursing facilities, and home care settings.
The telehealth solution combines clinically proven Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry with a cloud-based remote data capture and surveillance platform accessible from the patient's Android or iOS smartphone or smart device – or now, pre-installed on a Samsung Galaxy smartphone provided alongside the tetherless sensors. Monitoring key physiological data can help provide clinicians with an accurate snapshot of a patient’s respiratory status and facilitates awareness of the need for intervention.
Joe Kiani, Founder and CEO of Masimo, said, "Masimo SafetyNet is helping to save lives by ensuring patients remain monitored from afar even during quarantine. Older patients are at greater risk, but are less likely to have smartphones. Samsung reached out to us, wanting to help us with the pandemic. We are grateful for their help in making it easier and faster for our much needed technology to reach more of those patients who need remote monitoring and management the most."
Taher Behbehani, General Manager and Head of Mobile B2B for Samsung Electronics America, added, "Today, remote care solutions are more important than ever. We are proud to partner with Masimo to provide the technology and solutions our communities need. Our Galaxy smartphones with Knox security are fully equipped with the necessary connectivity and security to allow Masimo monitoring data to reach providers, safely and efficiently."
For added simplification and ease of use, the Samsung phones available alongside Masimo SafetyNet can be locked to provide only Masimo SafetyNet functionality.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Full Market Release of Masimo SafetyNet™, a Remote Patient Management Solution Designed to Aid COVID-19 Response Efforts
Secure Telehealth System Combines Tetherless Pulse Oximetry with a Remote Data Capture, Patient Surveillance, and Care Flow Platform
Irvine, California - April 10, 2020 – Masimo (NASDAQ: MASI) announced today the full market release of Masimo SafetyNet™, an innovative, economically scalable cloud-based patient management solution designed to help clinicians care for patients remotely in hospital settings and in non-traditional settings and circumstances. Successfully piloted at leading institutions like University Hospitals (UH) in Cleveland and St. Luke's University Health Network (St. Luke's) in Pennsylvania and New Jersey, Masimo SafetyNet is now available worldwide to help clinicians and public health officials combat the global COVID-19 pandemic.
|
|
On Wednesday, April 15 at 8:00 am PDT, Masimo and University Hospitals will jointly host a public webinar in which Dr. Peter Pronovost and his colleagues at University Hospitals join Masimo Founder and CEO Joe Kiani to discuss Masimo SafetyNet and UH’s experience using Masimo SafetyNet to help combat COVID-19. The webinar will cover best practices for deploying Masimo SafetyNet, based on UH's input and deployment of the platform, including the protocols they developed for their teams to integrate it into their response efforts. Clinicians and hospital staff worldwide are invited to participate and learn how they can deploy Masimo SafetyNet at their own institutions.
Masimo SafetyNet uses a tetherless, wearable single-patient-use SET® sensor to monitor a patient's blood oxygen saturation and respiration rate, as well as pulse rate, perfusion index, and PVi®, and is designed to help manage the surge in COVID-19 patients while maintaining distance from other patients and providers, allowing hospitals to expand patient remote monitoring into alternative care spaces, including overflow locations, emergency recovery facilities, and home care settings.
The telehealth solution combines clinically proven Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry with a cloud-based remote data capture and surveillance platform accessible from a patient's Android or iOS smartphone or smart device. Monitoring key physiological data can help provide clinicians with an accurate snapshot of a patient's respiratory status and facilitates awareness of the need for intervention.
"Many patients come to the hospital with COVID-19 symptoms and often times they are admitted simply because clinicians feel the patient would not be safe at home without regular monitoring," explained Peter Pronovost, MD, Chief Clinical Transformation Officer at University Hospitals in Cleveland, Ohio. "As such, many patients are admitted where they can potentially expose other patients and the staff to the coronavirus. With Masimo SafetyNet, we can now send patients home with the security of the patient and their provider knowing their critical vital signs such as oxygen saturation and respiration rate will be safely monitored at home. This technology also serves as a cost-effective way to scale monitoring and increase capacity for hospitals. We are currently using the product in the following ways: if a patient comes to the emergency department, but can be monitored at home rather than admitted; for patients who are discharged from the hospital and would still benefit from home monitoring; for patients being cared for by home health providers; and for cancer patients with COVID who would be at greater risk if cared for in an inpatient setting. And daily our clinicians are thinking of new ways to apply this technology."
Aldo Carmona, MD, St. Luke's Senior Vice President of Clinical Innovation and Chairman of the Department of Anesthesia and Critical Care, added, "I believe the collaboration of the St Luke’s and Masimo teams during our pilot of Masimo SafetyNet will improve the patient and provider experience. In conjunction with the Masimo team, we created a dashboard where multiple patients can be monitored in real time. This can be done rapidly on a floor where no previous monitoring existed and can be monitored from any location; alerts can go to providers in real time. After several years of using the Masimo Patient SafetyNet™ hard-wired system to help improve outcomes, Masimo SafetyNet was a natural solution for our network to meet capacity challenges during this pandemic."
In addition to COVID-19 CarePrograms, Masimo SafetyNet can be configured for more than 150 other CarePrograms, including COPD, heart failure, and oncology.
Joe Kiani, Founder and CEO of Masimo, said, "Masimo is proud to be able to support the brave nurses and doctors working around the clock to save lives across the world. We believe Masimo SafetyNet is a key asset in the fight against COVID-19, helping clinicians care for the extraordinary surge of patients as efficiently and effectively as possible, both in and out of the hospital. We are grateful for our partnerships with University Hospitals and St. Luke’s, whose piloting and early adoption of Masimo SafetyNet is already showing how this solution is making a difference. Everybody from UH, St Luke’s and Masimo has worked very hard over the past four weeks to improve the interface so that everyone can now benefit. In addition, we would like to thank our suppliers, Nordic Semiconductor and Texas Instruments, for prioritizing our orders of Masimo SafetyNet components, in volume, to help COVID-19 patients during this difficult time."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

St. Luke's University Health Network One of the First in the World to Pilot Masimo SafetyNet™, a Remote Patient Management Solution, to Aid Hospitalized COVID-19 Patients
Irvine, California and Bethlehem, Pennsylvania - April 7, 2020 – Masimo (NASDAQ: MASI) announced today that St. Luke's University Health Network (SLUHN) is one of the first institutions worldwide to use Masimo SafetyNet™ to monitor in-hospital patients, as the network seeks innovative solutions to care for the surge of patients infected by COVID-19. Masimo SafetyNet is an innovative, economically scalable cloud-based patient management platform designed to help clinicians care for patients remotely in hospital settings and in non-traditional settings and circumstances.
|
|
The telehealth solution uses a tetherless, wearable single-patient-use sensor to monitor patients with clinically proven Masimo SET® pulse oximetry, and is designed to help manage the surge in COVID-19 patients while maintaining the safety of other patients and providers, allowing hospitals to expand patient remote monitoring into alternative care spaces, including overflow locations, emergency recovery facilities, and home care settings.
Aldo Carmona, MD, St. Luke's Senior Vice President of Clinical Innovation and Chairman of the Department of Anesthesia and Critical Care, said, "This technology is game-changing in light of the crush of demand on our hospitals during this COVID-19 pandemic. With this wearable device, we can create temporary, pop-up respiratory monitoring units as needed to meet the changing patient volumes and track employees' health in their homes if they have been exposed to COVID-19, the flu, or any other serious illness."
Designed to track the blood oxygen saturation and respiration rate of patients who are hospitalized or quarantined at home, Masimo SafetyNet combines tetherless SET® pulse oximetry with a proprietary remote data capture and surveillance platform accessible from a patient's Android or iOS smartphone or smart device. Monitoring key physiological data can help provide clinicians with an accurate snapshot of a patient's systemic health and facilitates awareness of the need for rapid execution of treatment decisions that can be life-saving.
Patients are provided with a multi-day supply of single-patient-use sensors and access to the Masimo SafetyNet mobile app. With clinical feedback from St. Luke's led by Dr. Carmona and from University Hospitals led by Dr. Peter Pronovost, Masimo SafetyNet has been designed for easy, intuitive use to provide customized, interactive CarePrograms that align with expert guidance on COVID-19. Monitoring data collected by the sensor is shared with the patient’s smartphone using a secure Bluetooth® connection. Twice daily, or as directed, the CareProgram can be configured to actively notify patients to answer questions such as, "are you having trouble breathing?" and "what is your temperature?", and pushes these responses along with the monitoring data to clinicians for evaluation. CarePrograms are fully customizable to accommodate each institution's protocols, each patient’s needs, and any changes in COVID-19 guidance – and can be updated through the cloud by providers even after being deployed, for maximum flexibility as situations evolve.
In addition to COVID-19 CarePrograms, Masimo SafetyNet can be configured for more than 150 other CarePrograms for use with COPD, heart failure, oncology, and other patients.
On March 30, patients at St. Luke's University Health Network Bethlehem diagnosed with COVID-19 were outfitted with Masimo SafetyNet. Non-COVID-19 patients are also being monitored with this system in general medical-surgical units.
St. Luke's plans to use the Masimo SafetyNet tetherless sensor and cloud-based surveillance system to monitor upwards of 2,000 hospitalized patients and lower acuity cases in the home. These may also include staff and patients who are quarantined at home with the virus.
"Our patients at St. Luke’s have the most sophisticated and reliable respiratory monitoring available anywhere," Dr. Carmona says. "We know that continuous physiologic monitoring with Masimo’s Patient SafetyNet improves outcomes and saves lives. The ability to extend that capability to patients in non-traditional settings and at home during this crisis with Masimo SafetyNet is transformative. Only through our relationship with Masimo could this have been possible."
Joe Kiani, Founder and CEO of Masimo, said, "Masimo is proud to be able to work with St. Luke’s to help protect the health and safety of medical professionals and the patients they serve during this global pandemic."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About St. Luke's University Health Network
Founded in 1872, St. Luke's University Health Network (SLUHN) is a fully integrated, regional, non-profit network of more than 15,000 employees providing services at 11 hospitals and 300 outpatient sites. With annual net revenue greater than $2 billion, the Network’s service area includes 11 counties: Lehigh, Northampton, Berks, Bucks, Carbon, Montgomery, Monroe, Schuylkill, and Luzerne counties in Pennsylvania and Warren and Hunterdon counties in New Jersey. Dedicated to advancing medical education, St. Luke’s is the preeminent teaching hospital in central-eastern Pennsylvania. In partnership with Temple University, St. Luke’s created the Lehigh Valley’s first and only regional medical school campus. It also operates the nation’s longest continuously operating School of Nursing, established in 1884, and 34 fully accredited graduate medical educational programs with 263 residents and fellows. St. Luke’s is the only Lehigh Valley-based health care system with Medicare’s five- and four-star ratings (the highest) for quality, efficiency and patient satisfaction. St. Luke’s is both a Leapfrog Group and Healthgrades Top Hospital. In 2019, three of IBM Watson Health’s 100 Top Hospitals were St. Luke’s hospitals. St. Luke’s University Hospital has earned the 100 Top Major Teaching Hospital designation from IBM Watson Health seven times total and five years in a row. St. Luke’s has also been cited by IBM Watson Health as a 50 Top Cardiovascular Program. Utilizing the Epic electronic medical record (EMR) system for both inpatient and outpatient services, the Network is a multi-year recipient of the Most Wired award recognizing the breadth of the SLUHN’s information technology applications such as telehealth, online scheduling and online pricing information. St. Luke’s is also recognized as one of the state’s lowest cost providers.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SafetyNet™ and SET®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SafetyNet and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC's website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Media Contact: St. Luke's University Health Network
Sam Kennedy
Phone: (484) 526-4134
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Study Finds Zero Patient Deaths from Opioid-Induced Respiratory Depression Over 10 Years with Masimo SET® and Masimo Patient SafetyNet™ Technology
Irvine, California - March 25, 2020 – Masimo (NASDAQ: MASI), today announced the results of a recently published ten-year retrospective study in which researchers at Dartmouth-Hitchcock Medical Center investigated the impact of an integrated clinical surveillance monitoring system, using Masimo SET® and Patient SafetyNet™ technologies, on mortality related to the use of prescribed opioids in the general ward. Over the ten years studied, of 111,488 patients in units with surveillance available, there were zero patient deaths and no patients were harmed by opioid-induced respiratory depression while continuous monitoring was in use. In contrast, among patients in units without surveillance available, there were three deaths.1
|
|
The surveillance monitoring system provided continuous monitoring using Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, and was comprised of Masimo Radical-7® and Rad-87® Pulse CO-Oximeters®, Root® Patient Monitoring and Connectivity Hubs, and Masimo Patient SafetyNet™, which provided supplemental remote monitoring at central view stations and alarm and alarm escalation notifications to clinicians' pagers. Monitored parameters included oxygen saturation (SpO2) and pulse rate (PR). The researchers reviewed ten years of data collected from 2007 to 2017, over which time there were 126,697 general care unit discharges.
Dr. Sue McGrath and colleagues at Dartmouth-Hitchcock Medical Center found that, over the 10 years, of the 111,488 patients in units with surveillance monitoring available, "none died or were harmed by opioid-induced respiratory depression when surveillance monitoring was in use." Of the 15,209 patients in unmonitored units, three patients died from opioid overdose. The reduced death rate when surveillance was available, compared to when it was not available, was statistically "significant" (p=0.03).
A fourth patient died in a unit where surveillance monitoring was available but Masimo technology was not being used at the time of the adverse event. The researchers noted, "The fact that one patient with known risk for opioid sensitivity died while in a unit where monitoring was available but not in use highlights the importance of system adoption and adherence to standards of care."
The researchers concluded, "For a 10-year period, the rescue system with continuous surveillance monitoring had a profound effect on death from sedative/analgesic administration in the general care setting. This approach to patient safety can help address the risk of sedative/analgesic-related respiratory arrests in hospitals."Regarding the cost of deploying such a system, the researchers noted, "Although cost is often raised as a barrier to implementation, a previously performed financial analysis demonstrated cost-effectiveness of surveillance monitoring due primarily to intensive care unit patient days avoided when early detection of patient deterioration occurs."
They continued, "This study confirms that surveillance monitoring for pharmacologically induced respiratory arrest in hospitalized patients can virtually eliminate deaths due to this serious but treatable complication. In other high-risk, safety-focused industries, the level of evidence that currently exists for continuous surveillance monitoring to mitigate the risk of accidental sedative/analgesic overdose would likely prompt immediate calls for widespread implementation of safety interventions."
Joe Kiani, Founder and CEO of Masimo, commented, "We are incredibly grateful to Dr. McGrath, her colleagues, and everyone at Dartmouth-Hitchcock Medical Center for demonstrating the value of continuous monitoring of post-surgical patients over a ten-year period. Hundreds of other hospitals have adopted our technology and have reported similar results. We hope that this new study will inspire every other institution to implement Masimo SET® on their general floor to eliminate preventable deaths due to opioid overdose, especially at this time when illnesses that impact the respiratory system, such as COVID-19, are so prevalent."
In previously published studies at Dartmouth-Hitchcock Medical Center, researchers found that after deploying the continuous monitoring system in the original 36-bed unit, there was a 65% reduction in rapid response team activations and a 48% reduction in transfers back to the ICU.2 After five years of use, they reported zero preventable deaths or brain damage due to opioids, as well as cost savings of $7 million,3 and after ten years, they reported maintaining a 50% reduction in unplanned transfers and a 60% reduction in rescue events, despite increases in patient acuity and occupancy.4
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.8 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,9 improve CCHD screening in newborns,10 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.11-13 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,14 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.15 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, Doctella™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- McGrath S, McGovern K, Perreard I, Huang V, Moss L, Blike G. Inpatient Respiratory Arrest Associated with Sedative and Analgesic Medications: Impact of Continuous Monitoring on Patient Mortality and Severe Morbidity. J Patient Saf. 14 March 2020. DOI: 10.1097/PTS.0000000000000696.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb® and SpMet®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb and SpMet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

In Response to Blood Shortages Due to COVID-19, Masimo Offers Licenses for rainbow® Noninvasive Blood Constituent Monitoring, Including Total Hemoglobin, SpHb®, at No Additional Charge During the Coronavirus Pandemic
rainbow® Can Help Masimo Customers Better Manage Blood Supplies with SpHb® and Monitor Patients Receiving iNO Therapy with SpMet®
Irvine, California - March 23, 2020 – Masimo (NASDAQ: MASI), today announced that, in response to current worldwide blood shortages driven by the coronavirus pandemic, it is making rainbow® licenses available for no additional charge to hospitals where rainbow®-ready devices are already in use. The Masimo rainbow® platform allows for the noninvasive and continuous monitoring of 12 parameters, including hemoglobin (SpHb®), oxyhemoglobin (SpO2), and methemoglobin (SpMet®). Once rainbow® is enabled, hospitals can purchase RD rainbow® sensors at discounted prices during this pandemic. The program is available globally and is planned to continue until the pandemic subsides.
|
|
SpHb provides real-time visibility to changes, or lack of changes, in hemoglobin between invasive blood samples – and has been shown to help clinicians improve patient blood management. In multiple outcome studies, SpHb has been shown to help clinicians reduce blood transfusions.1-4 SpHb is available on a variety of Masimo Pulse CO-Oximeters®, including the Root® Patient Monitoring and Connectivity Platform, Radical-7®, and Rad-97®, as well as patient monitors from 25 other patient monitoring manufacturers, including Dräger and Philips.
"Our goal is to make the biggest difference we can during this challenging time. This is our third initiative in the past four days to help clinicians deal with COVID-19. Many hospitals have seen the value of rainbow®, and we hope every hospital can now benefit from proven rainbow® noninvasive blood constituent monitoring technology," said Joe Kiani, Founder and CEO of Masimo.
One of the many burdens COVID-19 is placing on health systems around the world is a shortage of blood products, as hospitals rush to treat an extraordinary surge of patients – at the same time that many blood drives have been cancelled. Therefore, hospitals are needing to manage blood supplies as efficiently and conservatively as possible. A growing body of evidence from around the world has shown that Masimo SpHb may help clinicians reduce unnecessary blood transfusions in both low- and high-blood-loss surgeries:
- • A randomized trial of 327 patients undergoing elective orthopedic surgery found that the use of continuous, noninvasive hemoglobin monitoring with SpHb reduced the rate of transfusions by 87% when compared to standard care without continuous, noninvasive hemoglobin monitoring.1
- • A prospective cohort study of 106 neurosurgical patients found that adding SpHb monitoring to standard-of-care blood management resulted in decreased blood utilization in high-blood-loss neurosurgery by 41%, while also decreasing time to transfusion when indicated by 41 minutes.2
- • A study of 100 patients undergoing abdominal cancer surgery found that SpHb monitoring decreased blood utilization by 39%, while facilitating earlier transfusions when indicated by 33 minutes.3
- • A study of 237 patients undergoing hip trauma surgery found that continuous SpHb monitoring during high-blood-loss surgery reduced the percentage of patients needing blood transfusions by 7% and number of transfused units per patient by 13%.4
In addition to SpHb, the rainbow® family of advanced noninvasive parameters includes SpMet, which helps clinicians noninvasively and continuously monitor methemoglobin levels in the blood.5 Elevated methemoglobin levels can be caused by inhaled nitric oxide (iNO) therapy,6-7 which is currently being investigated as a potential treatment for lung complications associated with COVID-19. By allowing clinicians to monitor methemoglobin levels, SpMet may be an important monitoring tool during iNO therapy.
SpHb and SpMet are not intended to replace laboratory blood testing. Clinical decisions regarding red blood cell transfusions should be based on the clinician's judgment considering, among other factors, patient condition and laboratory diagnostic tests using blood samples.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.8 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,9 improve CCHD screening in newborns,10 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.11-13 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,14 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.15 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, Doctella™, and Masimo SafetyNet™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Ehrenfeld JM et al. Continuous Non-invasive Hemoglobin Monitoring during Orthopedic Surgery: A Randomized Trial. J Blood Disorders Transf. 2014
- Awada WN et al. Continuous and noninvasive hemoglobin monitoring reduces red blood cell transfusion during neurosurgery: a prospective cohort study. J Clin Monit Comput. 2015 Feb 4. Study Protocol: The transfusion threshold of 10g/dL was predetermined by the study protocol and may not be appropriate for all patients. The blood sampling technique was the same for patients in both the control and the test group. Arterial blood was drawn from a 20-gauge radial artery cannula into 2mL ethylenediaminetetraacetic acid collection tubes, thoroughly mixed, then sent immediately to the central lab for analysis by a hematology analyzer. The reference laboratory device used for hemoglobin measurements in the study was a Coulter GEN-S Hematology Analyzer.
- Kamal AM et al. The Value of Continuous Noninvasive Hemoglobin Monitoring in Intraoperative Blood Transfusion Practice During Abdominal Cancer Surgery. Open J Anesth. 2016;13-19.
- Ribed-Sánchez B et al. Economic Analysis of the Reduction of Blood Transfusions during Surgical Procedures While Continuous Hemoglobin Monitoring is Used. Sensors. 2018, 18, 1367; doi:10.3390/s18051367.
- Annabi E et al. Severe Methemoglobinemia Detected by Pulse Oximetry. Anesth Analg. 2009 Mar;108(3):898-9.
- Riou Y et al. Pediatric Research. 1998. 43, 295-295.
- U.S. Food & Drug, Consumer Updates, Benzocaine and Babies: Not a Good Mix.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SpHb® and SpMet®. These forward-looking statements are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SpHb and SpMet, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo and University Hospitals Jointly Announce Masimo SafetyNet™, a New Remote Patient Management Solution Designed to Aid COVID-19 Response Efforts
Telehealth System Combines Radius PPG™ Tetherless Pulse Oximetry and the Doctella™ Platform
Irvine, California and Cleveland, Ohio - March 20, 2020 – Masimo (NASDAQ: MASI), and University Hospitals (UH), one of the largest health systems in Northeast Ohio, today jointly announced Masimo SafetyNet™, an innovative, economically scalable patient management system designed to help clinicians care for patients remotely.
The telehealth solution combines tetherless Radius PPG™ pulse oximetry, driven by breakthrough Masimo SET® Measure-through Motion and Low Perfusion™ technology, with Doctella™, a secure, home-based, remote patient surveillance platform accessible from a patient's iOS or Android smartphone or smart device.
The COVID-19 health emergency has significantly increased the demand for remote monitoring and patient engagement solutions in multiple settings. To proactively prepare for a surge in COVID-19 patients while maintaining the safety of other patients and providers, this new solution allows UH and other hospitals to expand patient monitoring to the home or to other facility locations set up temporarily to care for the increased demand. Current WHO guidelines recommend the monitoring of suspected or confirmed COVID-19 patients' oxygen saturation (SpO2), respiration rate (RR), and temperature, and Masimo and UH are meeting this increased demand by adapting existing technology to deliver a secure remote solution.
Masimo SafetyNet offers care teams a single-platform solution that couples a secure, cloud-based surveillance platform with clinically proven SET® pulse oximetry, estimated to be used on more than 200 million patients each year.1 In addition to SpO2, Radius PPG is capable of continuously monitoring pulse rate (PR), perfusion index, PVi®, and RRp®, respiration rate from the photoplethysmograph.
Patients can be sent home with a multi-day supply of single-patient-use Radius PPG sensors and access to the Doctella mobile app, designed for easy, intuitive patient use via a digital home-care plan, or CareProgram, that aligns with expert guidance on COVID-19. Radius PPG shares its SpO2, PR, and RRp data with Doctella using secure Bluetooth® wireless technology. In addition, Doctella can manually collect other physiological data, such as temperature. Twice daily, or as directed, the Doctella CareProgram actively notifies patients to answer questions such as, "are you having trouble breathing?" and "what is your temperature?", and securely pushes these responses along with physiological monitoring data to hospital-based clinicians for evaluation. The Doctella clinician portal allows providers to easily track patient compliance, helping them identify when intervention may be needed, as well as providing insight to help providers prioritize patients. CarePrograms are fully customizable to accommodate each institution’s protocols, each patient's needs, and any changes in COVID-19 guidance—and can be updated through the cloud by providers even after being deployed, for maximum flexibility as situations evolve.
"We appreciate Masimo’s immediate collaboration to stand up this innovative platform that will significantly aid in our ability to scale up remote monitoring and meet the demand for patient care while addressing capacity and safety issues," explained Peter Pronovost, MD, UH Chief Clinical Transformation Officer. "For our patients with congestive heart failure or chronic obstructive pulmonary disease (COPD), we can provide them with this remote monitoring capability so they do not risk a COVID-19 infection by being near potentially infected patients. Similarly, for patients with a confirmed COVID-19 diagnosis, we can appropriately isolate them from other patients while ensuring they have the access to state-of-the-art care for managing their health through the recovery process."
Joe Kiani, Founder and CEO of Masimo, said, "We thank the nurses and doctors who are bravely toiling in hospitals taking care of us all during this pandemic. We are delighted that we can help them triage and effectively take care of COVID-19 patients the best they can with Masimo SafetyNet. It has been a delight to work with UH to bring this technology to the health care industry to help them address the challenges they face during this unprecedented time."
"This alliance demonstrates UH's strategy to drive improvements in population health," said Daniel I. Simon, MD, UH Chief Clinical & Scientific Officer and President, UH Cleveland Medical Center. "By deploying 'outside-in' tactics and forming this partnership with Masimo, our patients receive cutting edge treatment approaches while we help enhance the product for future users and industry transformation."
UH plans to begin piloting this system next week.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About University Hospitals
Founded in 1866, University Hospitals serves the needs of patients through an integrated network of 18 hospitals, more than 50 health centers and outpatient facilities, and 200 physician offices in 16 counties throughout northern Ohio. The system's flagship academic medical center, University Hospitals Cleveland Medical Center, located in Cleveland's University Circle, is affiliated with Case Western Reserve University School of Medicine. The main campus also includes University Hospitals Rainbow Babies & Children's Hospital, ranked among the top children's hospitals in the nation; University Hospitals MacDonald Women's Hospital, Ohio's only hospital for women; University Hospitals Harrington Heart & Vascular Institute, a high-volume national referral center for complex cardiovascular procedures; and University Hospitals Seidman Cancer Center, part of the NCI-designated Case Comprehensive Cancer Center. UH is home to some of the most prestigious clinical and research programs in the nation, including cancer, pediatrics, women's health, orthopedics, radiology, neuroscience, cardiology and cardiovascular surgery, digestive health, transplantation and urology. UH Cleveland Medical Center is perennially among the highest performers in national ranking surveys, including "America’s Best Hospitals" from U.S. News & World Report. UH is also home to Harrington Discovery Institute at University Hospitals – part of The Harrington Project for Discovery & Development. UH is one of the largest employers in Northeast Ohio with 28,000 physicians and employees. Advancing the Science of Health and the Art of Compassion is UH’s vision for benefitting its patients into the future, and the organization’s unwavering mission is To Heal. To Teach. To Discover. Follow UH on LinkedIn, Facebook @UniversityHospitals and Twitter @UHhospitals. For more information, visit UHHospitals.org.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, those related to Masimo’s proposed acquisition of TNI medical AG (the "Acquisition"), and are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to achieving the conditions to closing the Acquisition and risks regarding the integration of assets acquired from TNI medical AG; risks relating to meeting customer demand or expectations of product success; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Media Contact: University Hospitals
George Stamatis
Phone: (216) 844-3667
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces Agreement to Acquire TNI medical AG
TNI Allows Masimo to Provide Its Customers with Innovative Pulmonary Care Therapy for Patients Suffering from Respiratory Insufficiency
Irvine, California - March 19, 2020 – Masimo (NASDAQ: MASI) a global leader in innovative noninvasive monitoring technologies, today announced it has entered into a definitive agreement to acquire TNI medical AG ("TNI"), an innovative ventilation company headquartered in Würzburg, Germany. In August 2019, Masimo disclosed an investment in TNI that included an exclusive option to acquire the company.
TNI's novel softFlow technology is designed to provide high flow, warmed and humidified respiratory gases to spontaneously breathing patients suffering from serious pulmonary conditions. The softFlow technology provides efficient, quiet and comfortable respiratory support by generating a precisely regulated, stable high flow of room air or a mix of room air and oxygen. The system, comprised of an integrated flow generator, respiratory circuit, and patient interface, operates without the need of pneumatic systems (i.e. compressed air supplied from the hospital wall) and can be used both in the hospital and at home.
Ewald Anger, CEO of TNI, said, "The TNI team is excited to join Masimo and leverage their deep engineering and commercial expertise to continue delivering our technology to a large and growing global market for therapy with nasal insufflation."
"We have been closely monitoring TNI since Masimo made its investment last summer and are thrilled to see the tremendous progress they have made in such a short time," said Joe Kiani, Founder, Chairman, and CEO of Masimo. "Due to COVID-19, there has been increasing demand for TNI's softFlow technology. We decided to exercise our option to acquire the company well before the option expired to allow Masimo to scale manufacturing. We believe this technology will provide clinicians with important additional tools to address the growing number of people affected by pulmonary diseases and respiratory-related illnesses, including those suffering from COVID-19. This acquisition illustrates our commitment to improving patient outcomes and reducing the cost of care."
The transaction is expected to close in the second quarter of 2020, subject to customary closing conditions. Masimo expects to fund the acquisition with existing cash on hand and expects the transaction to have an immaterial impact to net earnings in 2020.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 200 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, those related to Masimo’s proposed acquisition of TNI medical AG (the "Acquisition"), and are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to achieving the conditions to closing the Acquisition and risks regarding the integration of assets acquired from TNI medical AG; risks relating to meeting customer demand or expectations of product success; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo noninvasive medical breakthroughs provide cost-effective solutions and unique advantages; risks related to COVID-19; as well as other factors discussed in the "Risk Factors" section of our most recent reports filed with the Securities and Exchange Commission ("SEC"), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Investor Contact
Eli Kammerman
Phone: (949) 297-7077
Email:[email protected]
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo to Integrate Imprivata Medical Device Access Authentication Technology into Its Root® and Iris® Hospital Automation Platform
Irvine, California and Lexington, Massachusetts - March 10, 2020 – Masimo (NASDAQ: MASI) a global leader in noninvasive monitoring technologies, today announced a partnership with Imprivata®, the digital identity company for healthcare, to integrate Imprivata Medical Device Access – streamlined, secure authentication to medical devices for clinical users – into Masimo’s Hospital Automation solutions, featuring the Root® Patient Monitoring and Connectivity Platform and Iris Gateway®.
|
|
With the addition of Imprivata Medical Device Access to Masimo Root and Iris Gateway, hospitals gain access to an even more powerful patient monitoring and hospital automation solution designed to improve clinical workflows and efficiency in numerous ways. By allowing clinicians to instantaneously log on securely to medical devices, the enhanced solution will help them spend more time caring for patients without compromising the security of sensitive patient information.
"We are excited to work toward bringing Imprivata’s authentication technology to Masimo customers, to enhance their experience with our monitoring and automation solutions and to help them stay focused on improving patient outcomes. Our Root and Iris® platforms are designed to aggerate and automate the transfer of patient data as seamlessly as possible, to make sure clinicians have the most relevant, most timely information at their fingertips at all times," stated Bilal Muhsin, Chief Operating Officer, Masimo.
"In today's complex Internet of Medical Things, healthcare organizations are faced with the challenge of protecting the security and integrity of the information stored on and transmitted through medical devices without impeding clinical productivity,” said Wes Wright, Chief Technology Officer at Imprivata. "By partnering with Masimo, we're pleased to give our joint customers the ability to implement and enforce the security of sensitive information captured on Root and Iris platforms, without affecting clinical workflows."
Masimo Root is a powerful, expandable patient monitoring and connectivity hub that integrates an array of technologies, devices, and systems to provide multimodal monitoring and connectivity solutions in a single, clinician-centric platform. Root's plug-and-play expansion capabilities allow clinicians to centralize patient monitoring by bringing together advanced rainbow SET® parameters, brain function monitoring, regional oximetry, capnography, and vital signs measurements on an easy-to-interpret, customizable display, empowering clinicians with more information for making patient assessments.
Iris Gateway is a component of Masimo's Hospital Automation solution that provides third-party device integration and automates the transfer of data from medical devices into Electronic Medical Records (EMRs) to help improve productivity and reduce the likelihood of transcription errors.
Imprivata Medical Device Access is part of a comprehensive identity and multifactor authentication platform for fast, secure authentication workflows across the healthcare enterprise. Imprivata Medical Device Access combines security and convenience by enabling fast, secure authentication across clinical workflows while creating a secure, auditable chain of trust wherever, whenever, and however users interact with patient records and other sensitive data.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 150 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals listed in the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About Imprivata
Imprivata®, the digital identity company for healthcare, provides identity, authentication, and access management solutions that are purpose-built to solve healthcare's unique workflow, security, and compliance challenges. Imprivata enables healthcare securely by establishing trust between people, technology, and information across the increasingly complex healthcare ecosystem. For more information, please visit www.imprivata.com.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo Root® and Iris Gateway®. These forward-looking expectations are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo Root and Iris Gateway, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo’s unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Media Contact: Imprivata
Kerry Pillion
Phone:(781) 761-1452
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.

Masimo Announces FDA Clearance of Continuous RRp® Monitoring
Irvine, California - March 2, 2020 – Masimo (NASDAQ: MASI) announced today that continuous RRp® (respiration rate from the photoplethysmograph) monitoring of adult and pediatric patients with Rad-97®, Radical-7®, and Radius-7® Pulse CO-Oximeters® has received FDA clearance. With this clearance, both continuous and spot-check RRp are now available in the US, supported in a variety of pulse oximetry sensors and configurations, including the new non-cabled, tetherless, wearable Radius PPG™.
The availability of continuous RRp adds to Masimo's diverse portfolio of respiration rate monitoring modalities – acoustic respiration rate (RRa®), NomoLine® capnography (RRc™), and now photoplethysmographic respiration rate (RRp®) – to help clinicians ensure they have the right tools for each patient scenario.
Determining RR, or the number of breaths taken per minute, in many situations typically requires manually counting breaths with a timer and then converting to a rate per minute, or being fitted with chest leads or straps that can be inconvenient. Acoustic respiration rate, RRa, has been shown to be an accurate,1,2 reliable,1 easy-to-use,1 and easy-to-tolerate1,3 method of monitoring respiration rate on a continuous basis. If RRa or RRc is not available, however, for patients whose arterial oxygen saturation (SpO2) is already being monitored using Masimo SET®, RRp offers a convenient way to accurately obtain RR. RRp is particularly well suited to lower acuity settings like the general ward, where patients are less likely to have respiration rate monitoring technologies available. In scenarios where the ability to detect respiratory pause is important, such as during surgery or recovery after surgery, RRp should not be used; RRa or RRc is more appropriate.
With breathing difficulty generally considered one of the earliest signs of patient deterioration, Masimo hopes that the availability of RRp may be able to play a role in assisting clinicians and public health officials as they seek to combat respiratory-related illnesses, including the coronavirus COVID-19, especially when applying an additional sensor is not an option. In addition to RRp, RRc and RRa, Masimo offers a wide range of noninvasive and continuous monitoring technologies – including arterial oxygen saturation (SpO2), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), and total hemoglobin (SpHb®), as well as spot-check thermometry with the non-contact infrared thermometer TIR-1™ – designed to help clinicians monitor key physiological characteristics throughout the continuum of care, whether at the doctor's office, during triage in the emergency room, on the general floor, or in the ICU. To support these technologies, Masimo provides a variety of single-patient-use sensors (and non-contact technology in the case of TIR-1), which help mitigate the risk of patient-to-patient transmission of communicable diseases.
Alongside RRp, Masimo SET® sensors offer Measure-through Motion and Low Perfusion™ SET® pulse oximetry, which has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 SET® is estimated to be used on more than 150 million patients a year5 and is the primary pulse oximetry at 9 of the 10 hospitals that top the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.6
Joe Kiani, Founder and CEO of Masimo, said, "We aim to provide clinicians with the best monitoring tools so that they can provide the best care possible – which means recognizing that a monitoring method that works particularly well in one patient scenario may not be available or be the best choice in another. With the introduction of continuous RRp to our devices in the US, we are finally able to give clinicians in our home country a powerful third way to monitor respiration rate continuously, complementing other methods with a convenient and cost-effective single-sensor solution."
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.4 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,7 improve CCHD screening in newborns,8 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.9-11 Masimo SET® is estimated to be used on more than 150 million patients in leading hospitals and other healthcare settings around the world,5 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.6 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Macknet MR et al. Accuracy and Tolerance of a Novel Bioacoustic Respiratory Sensor in Pediatric Patients. Anesthesiology. 2007;107:A84 (abstract).
- Goudra BG et al. Comparison of Acoustic Respiration Rate, Impedance Pneumography and Capnometry Monitors for Respiration Rate Accuracy and Apnea Detection during GI Endoscopy Anesthesia. Open J Anesthesiol. 2013;3:74-79.
- Patino M et al. Accuracy of Acoustic Respiration Rate Monitoring in Pediatric Patients. Paediatr Anaesth. 2013 Sep 3.
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
Forward-Looking Statements
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo RRp®, RRa®, NomoLine®, RRc™, and SET®. These forward-looking expectations are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo RRp, RRa, NomoLine, RRc, and SET®, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo’s unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.
Masimo and MS Westfalia GmbH (MSW) Expand Partnership to Add Masimo SedLine® Brain Function Monitoring, O3® Regional Oximetry, and Oxygen Reserve Index (ORi™) to the MSW Modular Point-of-Care Hybrid Jenny
Jenny Combines Ventilation and Multi-Parameter Monitoring and Defibrillation in a Single Point-of-Care Device, Including Masimo rainbow SET® Measurements, NomoLine® Capnography, and now Brain Monitoring Technologies – The World's First All-in-One Solution of Its Kind
Irvine, California and Troisdorf, Germany - February 25, 2020 – Masimo (NASDAQ: MASI) and MS Westfalia GmbH (MSW) announced today that MSW will integrate additional Masimo measurement technologies into MSW's plug-and-play hybrid Jenny platform, to help clinicians assess brain function, oxygenation, ventilation, and resuscitation status.
After launching the Jenny modular point-of-care monitoring device with integrated Masimo noninvasive, continuous rainbow SET® measurements (including total hemoglobin, SpHb®) and Masimo sidestream and mainstream NomoLine® capnography, MSW plans to add Masimo Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and Oxygen Reserve Index (ORi™) to Jenny. By doing so, MSW will make the platform an even more versatile and comprehensive monitoring solution, suitable for use in a variety of care areas, including the ICU and the OR, as well as in EMS and military settings.
The three additional technologies, currently available directly from Masimo on the Root® Patient Monitoring and Connectivity Platform, are:
- Next Generation SedLine Brain Function Monitoring, which assists clinicians in monitoring the state of the brain under anesthesia, with bilateral data acquisition and processing of EEG signals and an enhanced Patient State Index (PSi).
- O3 Regional Oximetry, which may help clinicians monitor cerebral oxygenation in situations in which pulse oximetry alone may not be fully indicative of the oxygen in the brain.
- ORi, the first noninvasive and continuous parameter to provide insight into the oxygen reserve of patients receiving supplemental oxygen.
Eugen Kagan, CEO of MSW, said, "Our company's mission is to save lives and improve quality of life for our patients. For more than 25 years, our goal has been to develop products that assist healthcare providers in the hospital and pre-hospital markets by helping to improve patient outcomes and increase patient satisfaction and workflow efficiency – while lowering the cost of healthcare. We accomplish this by developing revolutionary approaches to new product development and by partnering with best-in-class partners like Masimo so we may meet the needs of a constantly changing healthcare landscape. The expanded technology partnership between MSW and Masimo allows us to offer our customers the most innovative technologies, helping them overcome many of the daily challenges they face in healthcare delivery."
Joe Kiani, Founder and CEO of Masimo, said, "It’s great to see MSW's commitment to incorporating our full suite of noninvasive, continuous measurements into their all-in-one modular Jenny platform. We are happy to be able to help MSW accomplish its wonderful mission."
ORi has obtained CE marking and is not available in the U.S.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
- Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
- Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
- de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
- Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
- Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
- McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
- Estimate: Masimo data on file.
- https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About MSW
MS Westfalia GmbH is one of the largest distributors of medical equipment on the market in Central Europe and the CIS. The company was founded in 1995. Since then, successful business allowed the company not only to develop the promotion of world famous brands, but also to begin its own production of high-tech medical devices.
Forward-Looking Statements: Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, statements regarding the potential effectiveness of Masimo SedLine®, O3®, ORi™, rainbow SET®, NomoLine®, and Root®. These forward-looking expectations are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo's unique noninvasive measurement technologies, including Masimo SedLine, O3, ORi, rainbow SET, NomoLine, and Root, contribute to positive clinical outcomes and patient safety; risks related to our belief that Masimo’s unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Media Contact: MSW
Julia Artel
Phone: +49 2241 944 933
Email: [email protected]
Improving Patient Outcomes and Reducing the Cost of Care®
Masimo to Acquire Connected Care Business from NantHealth
Irvine and Culver City, California - January 14, 2020 – Masimo (NASDAQ: MASI), a global leader in noninvasive monitoring technologies, and NantHealth, Inc. (NASDAQ: NH), a next-generation, evidence-based, personalized healthcare company, today announced the signing of a definitive agreement under which Masimo will acquire the Connected Care assets from NantHealth, Inc. for a $47.25 million upfront cash payment. NantHealth's Connected Care solutions provide medical device interoperability to hospitals and health systems. The portfolio includes DCX™ device connectivity (formerly known as DeviceConX™), VCX™ patient vitals software (formerly known as VitalsConX™), HBox® connectivity hub, and Shuttle interface cable.
The acquisition supports Masimo’s goal to help hospitals improve the continuum of great care through hospital automation, connectivity, and innovative noninvasive monitoring technologies. The Connected Care franchise is an established leader in providing connectivity solutions to hospitals, enabling streamlined collection and storage of medical device data through a vendor-agnostic platform into the EHR or other clinical information systems.
"One of the strategic priorities for Masimo is, through our Hospital Automation solutions, to reduce clinician cognitive overload and reduce errors of omission. Through connectivity, predictive algorithms, and decision support, we hope to improve the continuum of great care. The connectivity assets we are acquiring are completely in line with our mission as they will help accelerate our internal growth initiatives in this area," said Joe Kiani, Founder, Chairman, and CEO of Masimo. "Not only does Connected Care immediately increase our customer footprint but also provides us with products which complement our current portfolio. As a result, we are very excited to welcome Connected Care’s talented team to Masimo and look forward to realizing our vision together."
Patrick Soon-Shiong, MBBCh, MSc, FRCS (C), NantHealth Chief Executive Officer and Chairman of the Board, said, "Our decision to sell the Connected Care business enables us to focus on accelerating growth for our NaviNet® and Eviti® SaaS solutions, our data and molecular analytics capabilities, and pursue other strategically aligned goals. We believe Masimo has the best connectivity solutions, and we are delighted to have found the right home for our Connected Care business and our committed team of employees. We are working with Masimo to ensure a smooth transition for our Connected Care customers."
The transaction is expected to close in the first quarter of 2020, subject to customary closing conditions. Masimo expects to fund the acquisition with existing cash on hand. The estimated financial impact of the transaction is included in Masimo’s full-year 2020 financial guidance, which is being issued in a separate press release today.
About Masimo
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, patient monitors, and automation and connectivity solutions. Our mission is to improve patient outcomes and reduce the cost of care. Masimo SET® Measure-through Motion and Low Perfusion™ pulse oximetry, introduced in 1995, has been shown in over 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® has also been shown to help clinicians reduce severe retinopathy of prematurity in neonates,2 improve CCHD screening in newborns,3 and, when used for continuous monitoring with Masimo Patient SafetyNet™ in post-surgical wards, reduce rapid response team activations, ICU transfers, and costs.4-6 Masimo SET® is estimated to be used on more than 100 million patients in leading hospitals and other healthcare settings around the world,7 and is the primary pulse oximetry at 9 of the top 10 hospitals according to the 2019-20 U.S. News and World Report Best Hospitals Honor Roll.8 Masimo continues to refine SET® and in 2018, announced that SpO2 accuracy on RD SET® sensors during conditions of motion has been significantly improved, providing clinicians with even greater confidence that the SpO2 values they rely on accurately reflect a patient's physiological status. In 2005, Masimo introduced rainbow® Pulse CO-Oximetry technology, allowing noninvasive and continuous monitoring of blood constituents that previously could only be measured invasively, including total hemoglobin (SpHb®), oxygen content (SpOC™), carboxyhemoglobin (SpCO®), methemoglobin (SpMet®), Pleth Variability Index (PVi®), RPVi™ (rainbow® PVi), and Oxygen Reserve Index (ORi™). In 2013, Masimo introduced the Root® Patient Monitoring and Connectivity Platform, built from the ground up to be as flexible and expandable as possible to facilitate the addition of other Masimo and third-party monitoring technologies; key Masimo additions include Next Generation SedLine® Brain Function Monitoring, O3® Regional Oximetry, and ISA™ Capnography with NomoLine® sampling lines. Masimo's family of continuous and spot-check monitoring Pulse CO-Oximeters® includes devices designed for use in a variety of clinical and non-clinical scenarios, including tetherless, wearable technology, such as Radius-7® and Radius PPG™, portable devices like Rad-67™, fingertip pulse oximeters like MightySat® Rx, and devices available for use both in the hospital and at home, such as Rad-97®. Masimo hospital automation and connectivity solutions are centered around the Iris® platform, and include Iris Gateway®, Patient SafetyNet, Replica™, Halo ION™, UniView™, and Doctella™. Additional information about Masimo and its products may be found at https://www.masimo.com. Published clinical studies on Masimo products can be found at https://www.masimo.com/evidence/featured-studies/feature.
ORi and RPVi have not received FDA 510(k) clearance and are not available for sale in the United States. The use of the trademark Patient SafetyNet is under license from University HealthSystem Consortium.
References
1. Published clinical studies on pulse oximetry and the benefits of Masimo SET® can be found on our website at https://www.masimo.com. Comparative studies include independent and objective studies which are comprised of abstracts presented at scientific meetings and peer-reviewed journal articles.
2. Castillo A et al. Prevention of Retinopathy of Prematurity in Preterm Infants through Changes in Clinical Practice and SpO2 Technology. Acta Paediatr. 2011 Feb;100(2):188-92.
3.de-Wahl Granelli A et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;Jan 8;338.
4. Taenzer AH et al. Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: a before-and-after concurrence study. Anesthesiology. 2010:112(2):282-287.
5. Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
6. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
7.Taenzer A et al. Postoperative Monitoring – The Dartmouth Experience. Anesthesia Patient Safety Foundation Newsletter. Spring-Summer 2012.
8. McGrath SP et al. Surveillance Monitoring Management for General Care Units: Strategy, Design, and Implementation. The Joint Commission Journal on Quality and Patient Safety. 2016 Jul;42(7):293-302.
9. Estimate: Masimo data on file.
10. https://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
About NantHealth
NantHealth, a member of the NantWorks ecosystem of companies, provides leading solutions across the continuum of care for physicians, payors, patients and biopharmaceutical organizations. NantHealth enables the use of cutting-edge data and technology toward the goals of empowering clinical decision support and improving patient outcomes. NantHealth's comprehensive product portfolio combines the latest technology in payor/provider platforms that exchange information in near-real time (NaviNet and Eviti), connected care solutions that deliver medical device interoperability (DCX device connectivity platform and VCX patient vitals software) and molecular profiling services that combine comprehensive DNA & RNA tumor-normal profiling with pharmacogenomics analysis (GPS Cancer®). For more information, please visit www.nanthealth.com or follow us on Twitter, Facebook and LinkedIn.
Forward-Looking Statements: Masimo
This press release includes forward-looking statements as defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, in connection with the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, among others, those related to Masimo’s proposed acquisition of the Connected Care Business from NantHealth (the "Acquisition"), and are based on current expectations about future events affecting us and are subject to risks and uncertainties, all of which are difficult to predict and many of which are beyond our control and could cause our actual results to differ materially and adversely from those expressed in our forward-looking statements as a result of various risk factors, including, but not limited to: risks related to achieving the conditions to closing the Acquisition and risks regarding the integration of assets acquired from NantHealth; risks related to our assumptions regarding the repeatability of clinical results; risks related to our belief that Masimo’s unique noninvasive measurement technologies contribute to positive clinical outcomes and patient safety; as well as other factors discussed in the “Risk Factors” section of our most recent reports filed with the Securities and Exchange Commission (“SEC”), which may be obtained for free at the SEC’s website at www.sec.gov. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. All forward-looking statements included in this press release are expressly qualified in their entirety by the foregoing cautionary statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today’s date. We do not undertake any obligation to update, amend or clarify these statements or the “Risk Factors” contained in our most recent reports filed with the SEC, whether as a result of new information, future events or otherwise, except as may be required under the applicable securities laws.
Forward-Looking Statements: NantHealth
This news release contains certain statements of a forward-looking nature relating to future events or future business performance. Forward-looking statements can be identified by the words "expects," "anticipates," "believes," "intends," "estimates," "plans," "will," "outlook" and similar expressions. Forward-looking statements are based on management's current plans, estimates, assumptions and projections, and speak only as of the date they are made. Risks and uncertainties include, but are not limited to: our ability to successfully integrate a complex learning system to address a wide range of healthcare issues; our ability to successfully amass the requisite data to achieve maximum network effects; appropriately allocating financial and human resources across a broad array of product and service offerings; raising additional capital as necessary to fund our operations; achieving significant commercial market acceptance for our sequencing and molecular analysis solutions; establish relationships with, key thought leaders or payers' key decision makers in order to establish GPS Cancer as a standard of care for patients with cancer; our ability to grow the market for our Systems Infrastructure, and applications; successfully enhancing our Systems Infrastructure and applications to achieve market acceptance and keep pace with technological developments; customer concentration; competition; security breaches; bandwidth limitations; our ability to continue our relationship with NantOmics; our ability to obtain regulatory approvals; dependence upon senior management; the need to comply with and meet applicable laws and regulations; unexpected adverse events; clinical adoption and market acceptance of GPS Cancer; and anticipated cost savings. We undertake no obligation to update any forward-looking statement in light of new information or future events, except as otherwise required by law. Forward-looking statements involve inherent risks and uncertainties, most of which are difficult to predict and are generally beyond our control. Actual results or outcomes may differ materially from those implied by the forward-looking statements as a result of the impact of a number of factors, many of which are discussed in more detail in our reports filed with the Securities and Exchange Commission.
Investor Contact: Masimo
Eli Kammerman
Phone: (949) 297-7077
Email: [email protected]
Media Contact: Masimo
Evan Lamb
Phone: (949) 396-3376
Email: [email protected]
Media Contact: NantHealth
Kelly Neagu
Phone: (760) 760-0197
Email: [email protected]
Masimo, SET, Signal Extraction Technology, Improving Patient Outcome and Reducing Cost of Care by Taking Noninvasive Monitoring to New Sites and Applications, rainbow, SpHb, SpOC, SpCO, SpMet, PVI are trademarks or registered trademarks of Masimo.



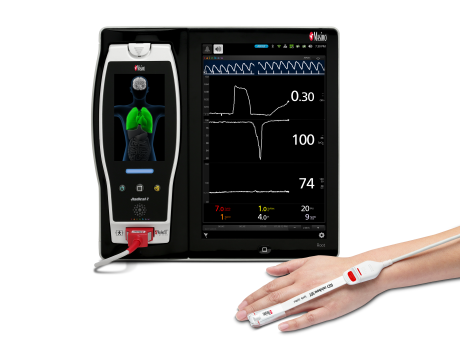




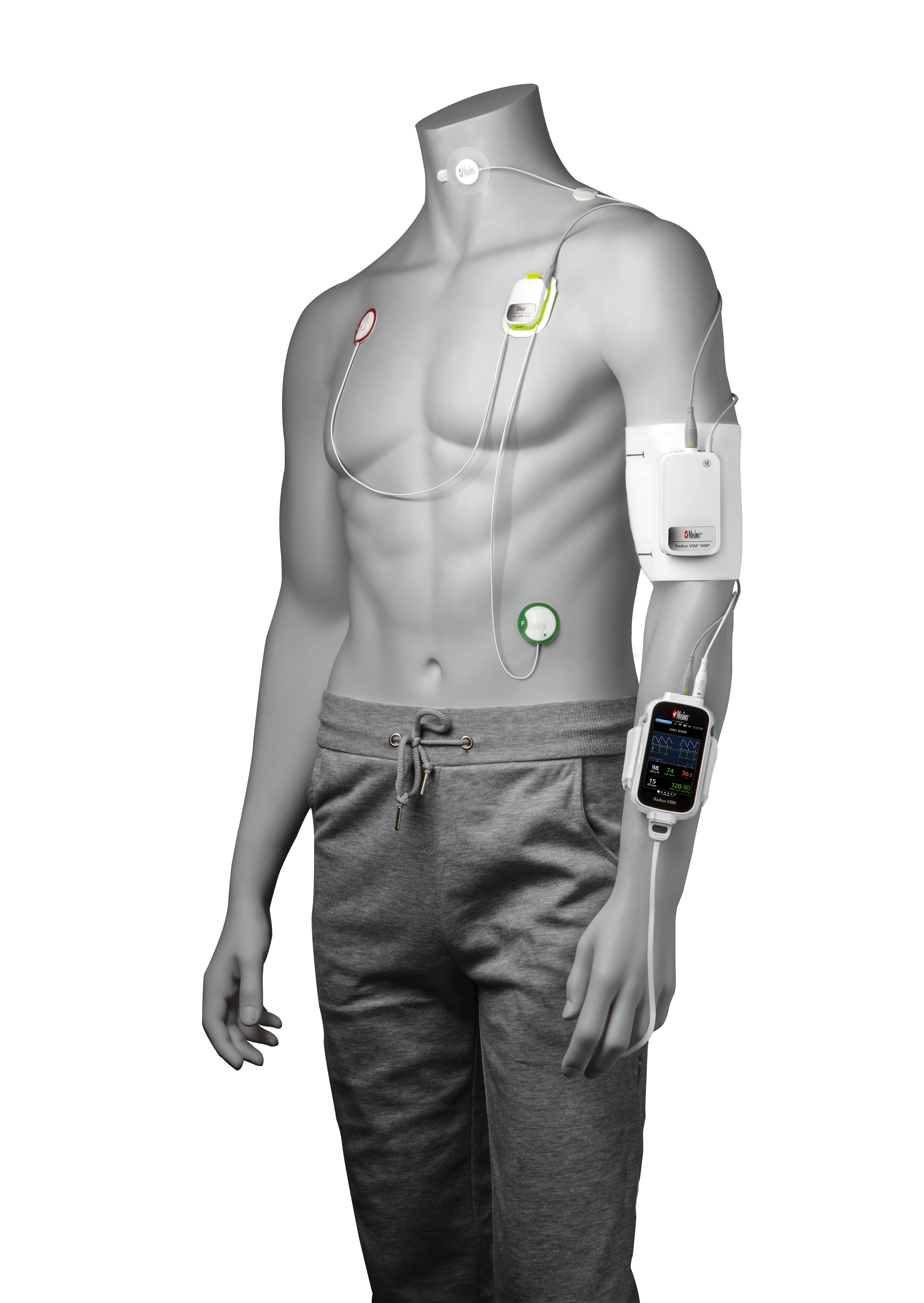



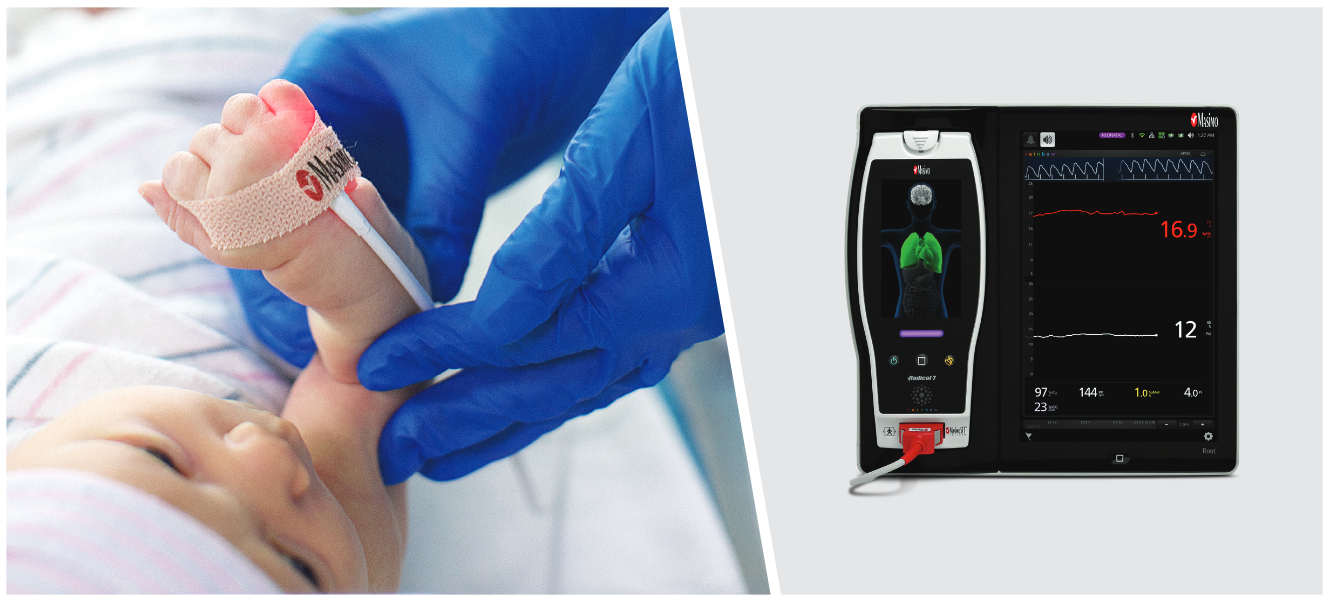

 CCHD Screening with Masimo SET® Pulse Oximetry
CCHD Screening with Masimo SET® Pulse Oximetry Masimo UniView: 60™
Masimo UniView: 60™ Masimo Rad-G™ with RRp®
Masimo Rad-G™ with RRp® Masimo Bridge™
Masimo Bridge™ Masimo Centroid™
Masimo Centroid™ Masimo SafetyNet-Open™
Masimo SafetyNet-Open™ Masimo Sleep™
Masimo Sleep™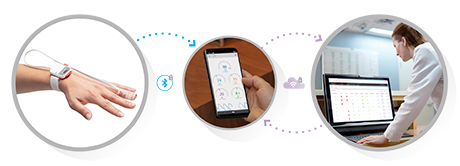 Masimo SafetyNet™
Masimo SafetyNet™ Masimo SafetyNet™
Masimo SafetyNet™